Introduction
Ecosystems exhibit complex relationships as numerous species interact with each other. In ecological networks, the species is represented by a node, and the relationships between the species are represented by links. The connecting links in the prey–predator food web represent the relationship between the prey and predator. In addition to the prey–predator network, there are various other network types, such as mutualistic networks and parasite–host networks (Bascompte, 2009; Bascompte et al., 2003; de Lima Filho et al., 2021; Dunne et al., 2002; Luz et al., 2021; McLeod & Leroux, 2021; Montoya et al., 2006; Strydom et al., 2021). In a mutualistic network, such as a seed disperser network or a plant–pollinator network, plants and animals are mutually supportive because they can profit from each other (Cohen, 2020; Hwang et al., 2008; Lee et al., 2012; Maeng & Lee, 2011; Olesen et al., 2007). However, in the parasite–host network, parasites benefit from the host. When a parasite parasitizes a host, the two species become linked.
Mutualistic relationships in ecosystems are classified into four types, namely seed dispersal, pollination, resource harvesting, and protection (Boucher, 1985). Plants that spread pollen via animals or insects use less energy to produce pollen, but they use considerable energy to produce colorful flowers and scents that attract animals and insects. Plants offer benefits to pollinators, including pollen, oil, resin, nectar, and fragrance; a common example is bees and flowers. Bees fly from one flower to another, gathering nectar. When they land on the flower, the bees obtain pollen as it rubs off onto their hairy bodies. When they land on the next flower, some of the pollen is rubbed off, thus pollinating the plant. In this mutualistic relationship, the bees consume the nectar, and the plants successfully reproduce.
In an ecological network, the degree of a node represents the number of connecting links. The degree of distribution for ecological networks characterizes the properties of ecological systems. When the degree of distribution function for the ecological network was investigated, various network types were identified. The cumulative degree of distribution function is defined as the integral of the degree distribution in the range of a specific lower degree to the maximum degree. The cumulative degree distribution functions identified in ecosystems have typical functions such as power law, exponential function, truncated scale-free function, stretched exponential function, uniform function, and irregular distribution (Lee et al., 2012; Maeng & Lee, 2011; Montoya et al., 2006). Table 1 summarizes the cumulative degree of distribution functions found in ecological networks.
Ecological networks have highly different characteristics compared to other complex networks. The total number of nodes in ecological networks is small because the ecosystems being examined by ecologists are relatively small (McLeod & Leroux, 2021; Montoya et al., 2006; Strydom et al., 2021). Given that ecosystems have a range of relationships between species, the characteristics of the connecting lines are distinct. Because species within an ecosystem strongly compete or cooperate with each other, the clustering coefficient is small compared with other networks. This clustering coefficient refers to the proportion of connections among the nearest neighbors of a node that are actually realized, compared with the number of all possible connections. A distinct hierarchy of predator relationships exists in the food chain. When the habitat environment in an ecosystem changes, the linkage or link strength between the species changes dynamically. Ecological networks are both robust and fragile. Some ecosystems maintain a high level of robustness against invasive species, whereas others are more vulnerable to rapid changes across the entire ecosystem (Maeng et al., 2019; Montoya et al., 2006).
In an ecological network, some species behave like wild bald eagles with many connections and are called generalists. However, some species are specialists with highly specific diets and a small number of prey species being consumed. Specialists have a fragile network structure because their survival cannot be guaranteed when connected species disappear, but they do have the advantage of being able to monopolize their prey. Research is currently underway to deepen our understanding of the expression principle of the structure of an ecological network. (Maeng et al., 2012; 2013; 2019). According to Maeng et al. (2019) in the case of a mutualistic network, the dependence of the cumulative degree of distribution function differs between plants and animals. The cumulative degree of distribution function of the plant–pollinator network has been recorded in coastal forests (Maeng & Lee, 2011).
Materials|Methods
Datasets on prey–pollinator networks
We examined datasets on plant–pollinator networks and suggested a model for growing them. We used plant–pollinator data from the Interaction Web Database ( http://www.ecologia.ib.usp.br/iwdb). After analyzing the structure of the network, we proposed a model that reproduces the structure of the observed mutualistic networks. We summarized the 14 plant–pollinator networks analyzed in this study. The number of nodes and links in the ecological network was small and the network was sparse.
Nonlinear evolutionary model of a mutualistic network
The principle driving the generation of the degree of distribution function in a mutualistic network is not well understood. Here, we developed a growth network model to reproduce the degree of distribution function observed in plant–pollinator networks. The degree of distribution function follows a power law or a stretched exponential distribution. Therefore, we considered the linear or nonlinear preferential attachment in bipartite-growing mutualistic networks. Fig. 1 shows a model of a growing bipartite mutualistic network. The network growth begins with the initial core network. During each update, we selected a plant with the probability qp and a pollinator with the probability qA=1–qp. The incoming node has links lP for the plant and links lA for the pollinator.
We controlled the connecting probability of the incoming links when a node such as a plant or a pollinator becomes attached to the existing network node. We then examined the linear and nonlinear preferential attachments for new incoming links. The linear preferential attachment introduced by Barabasi and Albert (1999), known as the Barbasi–Albert model, exhibits a scale-free degree distribution. The nonlinear preference attachment model can explain the stretched exponential function of the degree of distribution (Krapivsky & Redner, 2001; Maeng et al., 2019). We applied the general nonlinear preferential attachment of incoming links for plants and pollinators. The incoming links for the plant or pollinator were chosen as the target nodes according to the nonlinear preferential attachment with an attaching probability of for the pollinators and for the plants. We then repeated the attachment process to reach the target network size.
Results|Discussion
Solutions for a nonlinear evolutionary model of a mutualistic network
The growing mutualistic network follows the power law of the degree of distribution as λA or P=1. When the parameter is less than one (0<λ<1), the degree of distribution of the plant or pollinator shows a stretched exponential function expressed as P(k)~exp(–k1–λj), where j=A or P. We set a master equation for the mean number of plants and pollinators. Maeng et al. (2012) obtained the degree of distribution for the growing networks. For λX=1, the degree of distribution follows the power law:
where the power-law exponent is obtained as and X=(A or P). For λX<1, the degree distribution shows a stretched exponential function such that
where .
Structure of growing bipartite mutualistic networks
We generated the growing bipartite mutualistic networks from the initial core networks for a given set of parameter sets (PA,PP,lA,lP), as shown in Fig. 1. We simulated a mutualistic network using linear or nonlinear growth exponents for preferential attachment. Fig. 2 shows the cumulative degree of distribution of the simulated growing bipartite mutualistic network with nonlinear exponents for λA=1.0 for the plant and λP=0.5 for the animal and for the given set of parameter sets ( ). The cumulative degree of distribution shows the power law for the animal with λA=1.0 and the stretched exponential distribution for the plant with λP=0.5. The simulated results were consistent with the analytical prediction.
Applications of the model for growing bipartite networks to real mutualistic networks
We applied the model of the growing bipartite mutualistic network to 14 empirical plant–pollinator networks. The incoming number of links lP for plants is much larger than that of the animal lA. There were found to be more plants than animals in the mutualistic networks, as shown in Table 2; the nonlinear growth exponents show significant asymmetry. In most of the networks in Table 2, we recorded the relation λA>λP except in the case of the Hocking network. This implies a significantly strong competition between a new animal and existing pollinators, in contrast with the relatively weak competition between plants. The restriction on the number of available plant species is a more important factor in shaping the mutualistic community than the restriction on the animal species available, which is likely related to the difference in their survival and reproduction rates. Plants with a large degree of distribution have the advantage of high abundance, and are screened by the competition between animals characterized by λP<1, which leads the degree of distribution to take the stretched exponential form. This is not the case for “Hocking” in Fig. 3, where it has been reported in Hocking (1968) that competition between plants is more significant than that between the pollinators, implying λA>λP .
In summary, we determined the degree of distribution for the plant–pollinator network and introduced a growing model for bipartite mutualistic networks. We can reproduce the power law or stretched exponential degree of distribution for a mutualistic network for plants and animals. Thus, we report that competition among animals is stronger than that among plants.
Figures and Tables
Fig. 1
Model of a growing bipartite network. The growth of the network starts from the initial core network. During each update, we selected a plant with a probability Pp and a pollinator with a probability PA=1– Pp. The incoming node has links lp for the plant and links lA for the pollinator.
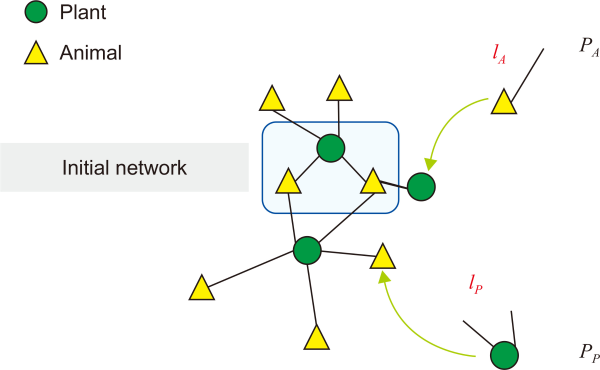
Fig. 2
Simulation of a growing bipartite mutualistic network using the growing exponents λA=1.0 for the plant and λP=0.5 for the animal and for the given set of parameter sets ( ). We plotted the cumulative degree of distribution for the simulated results (symbols) and the analysis results (the dashed and dot-dashed line).
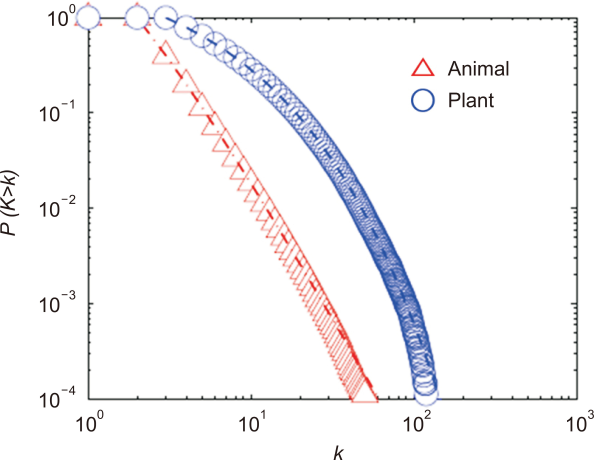
Fig. 3
Plot of the growth exponent λA for the plant versus λA for the empirical mutualistic networks. The nonlinear growing exponents (λA, λP) are obtained by fitting the cumulative degree of distribution of the empirical plant and pollinator, respectively.
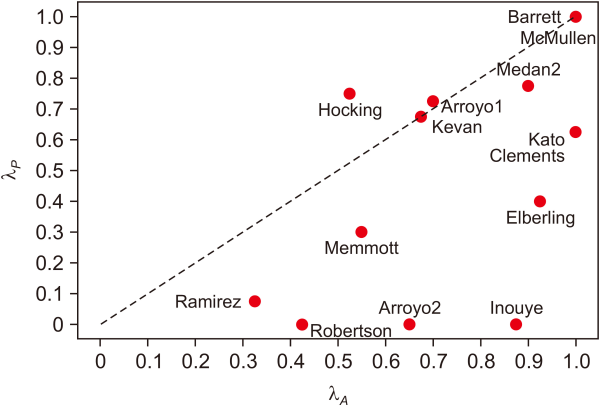
Table 1
Types of cumulative degree of distribution in the ecological networks
| Function | Cumulative degree distribution |
|---|---|
| Power law | Pc(k)~k–γ |
| Exponential function | Pc(k)~exp(–ak) |
| Truncated power law | Pc(k)~k–γexp(–k/γ) |
| Stretched exponential function | Pc(k)~exp(–akβ) |
| Uniform function | Pc(k)~constant |
| No functional form | Irregularly distributed |
Table 2
Fourteen plant–pollinator networks
| Network | Animals | Plants | Links | Reference |
|---|---|---|---|---|
| Arroyo1 | 87 | 98 | 371 | Arroyo et al. (1982) |
| Arroyo2 | 43 | 62 | 199 | Arroyo et al. (1982) |
| Barret | 12 | 102 | 167 | Barrett and Helenurm (1987) |
| Clements | 96 | 275 | 923 | Clements and Long (1923) |
| Elberling | 23 | 118 | 238 | Elberling and Olesen (1999) |
| Hocking | 29 | 86 | 184 | Hocking (1968) |
| Inouye | 42 | 91 | 281 | Inouye and Pyke (1988) |
| Kato | 93 | 679 | 1,206 | Kato et al. (1990) |
| Kevan | 32 | 115 | 312 | Kevan (1970) |
| McMullen | 106 | 54 | 204 | McMullen (1993) |
| Medan | 23 | 72 | 125 | Medan et al. (2002) |
| Memmott | 25 | 79 | 299 | Memmott (1999) |
| Ramirez | 33 | 53 | 109 | Ramirez and Brito (1992) |
| Robertson | 456 | 1,428 | 15,255 | Robertson (1928) |