Introduction
Rats are a small species of mammals that occupy the lower trophic level of the food web, and are food sources for various predator species. Species of the genus Rattus living in Korea include R. norvegicus, R. rattus, and R. tanezumi (Jo et al., 2018). In particular, R. norvegicus (called the brown rat or Norway rat) is a relatively large species of the genus Rattus, with a total length of 302-355 mm, tail length of 140-167 mm, and weight of approximately 90-500 g. Brown rats are present nationwide, in habitats including islands, farmlands, and forests (Jo et al., 2018). Kang et al. (2008) and Oh et al. (2008) also observed them on islands. Brown rats are omnivorous and have a lifespan of four years in captivity or only 1-2 years in the wild.
According to the IUCN Red List, the Chinese crested tern (Thalasseus bersteini) is an extremely rare and globally critically endangered species. This species is listed as “Critically Endangered (CE)” on the IUCN Red List, and the surviving population is estimated to consist of approximately 30-49 individuals (BirdLife International, 2021). Prior to the discovery of a breeding site on the western coast of South Korea, the only known breeding sites were in China (Jiushan and Wuzhishan) and Taiwan (Matzu and Penghu Islands) (Chen et al., 2009). Currently, five described breeding sites remain (Song et al., 2017). Historically, the breeding populations in China have been pushed to the brink of extinction due to egg poaching, breeding failure due to typhoons, marine pollution, and hybridization with the Great crested tern (Chan et al., 2010). Yuksan Island, Korea, was declared a marine sanctuary (Natural Monument No. 389) to protect the breeding grounds of endangered marine bird species. Moreover, it was declared a Specified Island (No. 239) after Chinese crested tern breeding was confirmed during a census to investigate the natural habitats of uninhabited islands. In addition, the breeding period (late March-early July) of Chinese crested terns does not overlap with the typhoon season, which greatly reduces the risk of natural hazards (Ministry of Environment, National Institute of Ecology, 2019). Furthermore, the breeding colony of the Chinese crested terns being located close to breeding colonies of the distant Black-tailed gulls (Larus crassirostris) suggests a low risk of hybridization. Hybridization is known to be a great risk to Chinese and Taiwanese Chinese crested tern populations, but it does not seem to be significantly important to the South Korean population. However, Yuksan Island may have different risk factors for this species. Therefore, research and monitoring of the factors influencing the breeding of avian species on this island are of crucial importance.
Generally, goats, rabbits, felines, and rodents may threaten the breeding grounds of avian species (Russel & Le Corre, 2008). In particular, the impact of rodents has frequently been reported in the literature (Jones et al., 2008; Lee & Yoo, 2002). Previous censuses reported that the population size of brown rats (R. norvegicus) is approximately 50-100 (Cultural Heritage Administration, 2004) on Yuksan Island, the only breeding site of the Chinese crested tern in South Korea. Hence, the presence of brown rats may threaten the breeding of Chinese crested terns in South Korea. The impact of rodents on marine birds has been investigated from various perspectives (Howald et al., 2007; Møller, 1983; Towns et al., 2006). For example, on Sasu Island in South Korea, the presence of brown rats accounts for 85% of all hatching failures in the Streaked shearwater (Calonectris leucomelas) (Nam et al., 2014). However, it has also been reported that gulls (Larus spp.) may feed on rodents; thus, it is even more necessary to elucidate the prey–predator relationship between Chinese crested terns and brown rats on Yuksan Island. Moreover, Jones et al. (2008) reported that small species within the Hydrobatidae, or even smaller species, and especially burrow-nesting species, may be largely influenced by rodents, whereas larger species within the Laridae and ground-nesting species may be less influenced. The Chinese crested tern is a ground-nesting species, but it is smaller than many other species belonging to the Laridae family, making it potentially vulnerable to the presence of brown rats.
Atoms consist of neutrons, protons, and electrons, with neutrons and protons tending to exist in identical quantities. However, some atoms have one more neutron than protons, known as isotopes. There are two different types of isotopes: radioisotopes, with radioactivity, and stable isotopes, such as C, N, O, and P, which are used in stable isotope analyses and tend to have one more neutron than the number of protons. Owing to these characteristics, stable isotopes may accumulate within organisms during activity. Stable isotopes (e.g., 13C and 12C), which are constantly used during biological activities and are also absorbed from food sources, have been shown to have similar ratios across organisms that are in prey–predator relationships. Empirical studies using stable isotope analyses for food source analyses have been performed (Hong et al., 2016; Major et al., 2007; Tabak et al., 2016). In the case of 15N, which is depleted less during feeding activities than C and consequently accumulates, it has been shown that predators have higher nitrogen values than their prey; thus, it is a highly valuable stable isotope in studies on prey–predator relationships. In this study, we predicted the carbon sources of terrestrial and marine ecosystems would be different, and that we would be able to determine whether the brown rat feeds on birds or eggs through the 13C level.
This study aimed to assess the potential threats that brown rats may pose to the Chinese crested tern by investigating the population status and feeding behavior of brown rats on Yuksan Island, and determine the carbon source of the food sources of the brown rat by conducting stable isotope analysis.
Materials|Methods
Study area
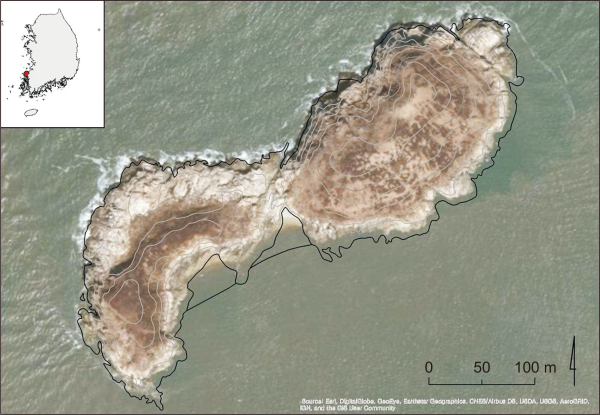
The study area is Yuksan Island, with a surface area of approximately 50,000 m2, in Jeonlanam-do (Province), Yeonggwang-gun (County), approximately 14 km offshore of Kyema Port in Yeonggwang-gun. Chinese crested tern breeding on Yuksan Island was reported for the first time in April 2016 when conducting a natural habitat census on uninhabited islands. The Ministry of Environment declared the island as Specified Island No. 239 on February 13, 2017, and hence prohibited the public from accessing or conducting any activities on the island to protect the breeding grounds of these Chinese crested terns. Therefore, the South Korean breeding site for Chinese crested terns is an ecologically well-protected area without any human activity. Yuksan Island is an important habitat for many marine bird species, including the endangered Black-faced spoonbill (Platalea minor) and the Chinese egret (Egretta eulophotes), and is also the main breeding ground for the Black-tailed gull (Larus crassirostris). The northeastern pinnacle of the island is 38 m above sea level the southwestern pinnacle is 22 m above sea level, and the middle part connecting these two pinnacles is always above sea level, even during high tide (Fig. 1). There is a sandy beach on the southwestern part of the island that serves as resting and hunting grounds for birds during low tide, and the rest of the island consists of rocky areas. Yuksan Island largely consists of rocky walls, rocks, and naked ground with few plants.
The vegetation on Yuksan Island varies across the life-cycle of the avian species. There is little vegetation until July, the group breeding season for avian species. Most of the surface of Yuksan Island is used by Black-tailed gulls for breeding, and vegetation gradually grows from August onwards, when Black-tailed gull chicks are fledged (Ministry of Environment, National Institute of Ecology, 2019; 2020). We mainly observed Indian goosegrass (Eleusine indica) and Coast rock sedge (Carex boottiana), along with some tree species (e.g., Euonymus japonicus, Vitex rotundifolia, Albizia julibrissin, and Clerodendrum trichotomum). From March to June, C. boottiana dominates the landscape of Yuksan Island, while E. indica starts forming large colonies on initially naked land near the breeding colonies from July onwards.
Samples
To investigate the feeding status of brown rats on Yuksan Island, we sampled brown rats as well as animals and plants that we predicted that brown rats would feed upon (Table 1). To catch brown rats, we used rat traps and lured them with dried sardines (Sardinella zunasi). Moreover, we collected fruits, edible plants producing seeds, and insects that could be distinguished by the naked eye. To consider the impact on the breeding of marine birds, we also sampled Black-tailed gull adults that died for unknown reasons and abandoned eggs. Although it would have been beneficial to sample Chinese crested terns to determine the impact on Chinese crested terns, we could not because of their extremely low population size. Among the 28 samples, we selected 16 that were considered to be in a direct predator–prey relationship with the brown rat (four Black-tailed gull adults and one egg sample, five brown rat samples, three plant samples, and three insect samples, but no Chinese crested tern samples) and conducted stable isotope analyses. All the samples were cleaned with 100% EtOH prior to stable isotope analysis. All samples were collected from Yuksan Island, located in Yeonggwang-gun, for 18 months from March 2019 to September 2020. All samples were sent to the Seoul Cooperative Center of Hanyang University to prepare tin capsules for stable isotope analyses using elemental analysis-isotope ratio mass spectrometry (EA-IRMS).
Food source analysis
A prior census of their nests is necessary to catch brown rats for food source analysis. Hence, we attempted to find rat burrows with the naked eye. We selected 20 potential catch points based on our prior census of feces in the rat burrows and set automatically triggered camera and rat traps (19×9×11 cm) on the points. For stable isotope analysis, fresh samples of living organisms were collected whenever possible. However, owing to the low accessibility of the study area, this was not always feasible. Therefore, brown rat bodies were collected within 3 days of death. Femurs, caudal vertebrae, and fresh muscle tissues were used as samples (fresh within the last 1-2 days after death). In the case of plants, we mainly focused on edible materials (e.g., fruits and seeds). We also sampled the roots of inedible plant species. In insects, we ground the entire organism for analysis. The samples were freeze-dried for five consecutive days. The dried samples were then autoclaved and subsequently placed in 2-mL tubes with stainless beads washed with 100% EtOH. The tubes were placed in a TissueLyser II (Cat. No. 85300; Qiagen, Hilden, Germany) and ground for 120 seconds at a speed of 1,680 rpm. The Seoul Cooperative Center of Hanyang University was consulted to determine the 13C and 15N ratios of the powdered samples. Stable isotope analysis results were analyzed using dot graphs in Microsoft Excel (Microsoft, Redmond, WA, USA).
To increase the accuracy of our analysis, we collected fecal samples from brown rats and analyzed the food sources that remained undigested within the feces. For this analysis, we chose fecal samples that were less than 1 day old and had retained their original shape and color. When fecal samples from brown rats were found, we recorded the coordinates and placed the samples in polyethylene containers. The fecal samples were stored at –20°C until further analysis. To determine the food materials, each fecal sample was placed in a 50-mL conical tube, subsequently combined with distilled water, and filtered using a 500-µm mesh. The filtered materials were dried in a Petri dish and observed under a microscope to determine the food materials.
Results
Stable isotope analysis
The 13C values that provided information regarding the food source were separated between terrestrial and marine ecosystems. Insect and Indian goosegrass samples, which were expected to serve as food items for the brown rat from the terrestrial ecosystem, had similar 13C values of –16‰ to –11‰, while the 13C value of the Black-tailed gull samples, which were expected to feed on the items obtained from the marine ecosystem, was approximately –22‰ to –18‰ (Fig. 2). Interestingly, the 15N value of the plant material, which was supposed to be a producer at the bottom of the food chain, was higher than that of the Black-tailed gull, a predator.
Food sources of the brown rat
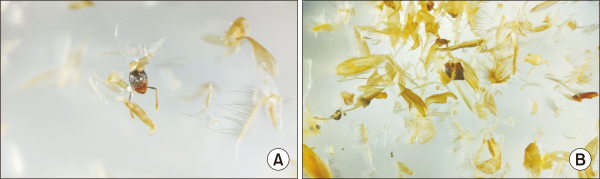
To confirm the results obtained by the stable isotope analysis, we additionally examined the undigested food items within the fecal samples of brown rats (Fig. 3). By examining the feces of brown rats, we confirmed that it mainly consisted of plant material, such as plant seeds, and the proportion of animal food sources, such as insects, was relatively low.
Discussion
This study analyzed factors that may threaten the breeding of avian species, including the globally endangered Chinese crested tern. In particular, brown rats inhabiting Yuksan Island may directly threaten the breeding of avian species; therefore, the breeding and feeding status of these brown rats should be thoroughly monitored for the management of Yuksan Island. For this purpose, we investigated the distribution of brown rat burrows in the field and sampled brown rat tissues, as well as edible plant and animal materials that may serve as food sources for brown rats, to conduct stable isotope analyses. We used the tail bones of the rats to determine their main food source. The avian species breeding on Yuksan Island stay for about half a year, while rats have a lifespan of 1-2 years; hence, about half of their lives overlaps with the avian species’ presence on the island. Thus, this duration may be considered sufficient for stable isotopes from avian species to accumulate in rat bones.
Additionally, we analyzed undigested food sources from the fecal samples under a microscope.
From the 13C values that provided information on the food source, which we separated into terrestrial and marine ecosystem sources, we deduced that brown rats mainly relied on food sources taken from the terrestrial environment, such as insects and flowering Indian goosegrasses, which had similar 13C values of –16‰ to –11‰. The 13C value of the Black-tailed gull, which we expected to feed on items obtained from the marine ecosystem, was approximately –22‰ to –18‰ (Table 2). Insects and flowering Indian goosegrasses are not in direct prey–predator relationships with Black-tailed gulls and Chinese crested terns, which feed on items obtained from the marine environment. A few rats had a similar carbon level to black-tailed gulls, but the possibility that they fed on similar food sources taken from the marine environment (e.g., stranded fish brought in by waves) is higher than the possibility that rats fed directly on Black-tailed gulls or Chinese crested terns. The stable isotope values from the bone of the tip of the tail in the rats were approximately –11.5‰ and –13.08‰. These values were even more different to those of Black-tailed gulls.
The 15N value of the plant material, which was supposed to be a producer at the bottom of the food chain, was even higher than that of the Black-tailed gull, which is a predator. This difference may have been due to the sampling period. The flowering period of Indian goosegrass is two months after Black-tailed gulls leave their breeding sites; hence, the guano is absorbed by the soil and the nitrogen level of the soil is the highest. The seeds of the plants were more nutritious than the bodies, and their constituents differed greatly. They accumulate nitrogen to sprout; therefore, it could be deduced that the nitrogen level of the plant material was the highest during this period. Generally, the 15N value of plant material from terrestrial ecosystems does not exceed 10 (Chahartaghi et al., 2005). In this study, we found that the 15N values of plants exceeded 18 (Craine et al., 2015; Xu et al., 2010; Yan et al., 2020). This may be due to external factors enriching the nitrogen of the soil on this island, such as the guano of Black-tailed gulls. The study site, Yuksan Island, is the breeding site of approximately 30,000 Black-tailed gulls that feed on items obtained from the marine environment over a relatively long period; hence, the guano of the Black-tailed gulls likely enriches the 15N values of the soil on this island. Plant material rarely leaves the island, but the external factors enriching nitrogen are maintained constantly, and very little nitrogen is washed away by rainwater.
To confirm the results obtained by our stable isotope analysis, we examined the undigested food sources within the fecal samples of brown rats (Fig. 3). Much of the undigested food consisted of plant material, such as plant seeds, and the proportion of animal matter, such as insects, was relatively low. Considering that food sources found in fecal samples are the remaining undigested, low-quality parts of the initial food consumed, this cannot be considered an absolute result, but is rather a supplement adding to the results of our stable isotope analysis. Feeding conditions of live organisms are determined by nutrition, handling time, and frequency (Pulliam, 1974). As high-quality food sources of animal matter are scarce on the island, brown rats seemed to have fed more frequently on the readily available plant materials. Moreover, due to the length of the gut and the short digestion time of the rat, they probably cannot digest and absorb all plant materials readily, which may explain the high content of undigested plant material in the feces.
Research on food sources and chains always entails certain types of errors. Regardless of whether the intake of certain food sources was high or not, if it was easily digested, it may not have been observed in fecal samples. In addition, even if the intake was low, hardy sources could pass through without being digested, and therefore may impart bias upon the main food source. To account for such limitations, we conducted stable isotope analysis, but it was not feasible to collect sufficient samples of all plant and animal species inhabiting the island due to its characteristics. A future study including sufficient samples of algae and fish species from the marine ecosystem will be able to provide a much clearer result.
In conclusion, contrary to our hypothesis that brown rats would feed on birds and eggs, which are excellent sources of protein, we found that there was no direct effect of brown rats on avian species. However, this study was limited to a small set of food sources, which may have imposed a bias. Therefore, future studies considering a wider array of samples, including plant and animal species inhabiting both the terrestrial and marine ecosystems of this island, will be necessary to assess the impact of brown rats on the breeding colony of avian species. Furthermore, environmental factors may push brown rats to change their feeding behavior, rendering it even more crucial to continue monitoring them.
Figures and Tables
Fig. 2
Food items in brown rat scats. (A) Animal food sources observed in August 2020. (B) Plant food sources observed in October 2020.
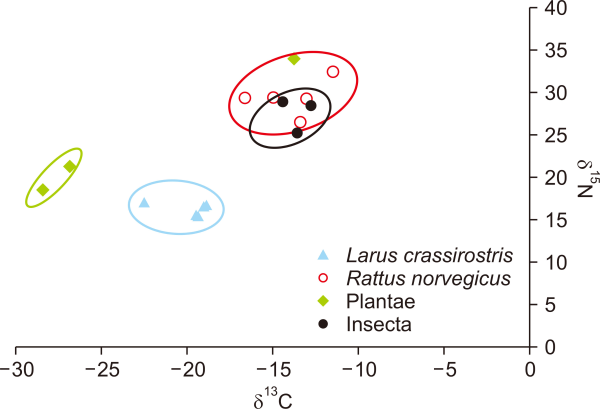
Table 1
The list of brown rat sample
| No. | Trapping date | Sampling date | Sex | Sample type | Etc. |
|---|---|---|---|---|---|
| 1* | - | 2019. 3. 18 | Unknown | Corpse | |
| 2* | 2019. 8. 28. - 2019. 9. 2. | 2019. 9. 2. | Unknown | Trap | |
| 3* | 2019. 8. 28. - 2019. 9. 2. | 2019. 9. 2. | Unknown | Trap | |
| 4* | - | 2019. 10. 10. | Unknown | Corpse | Juvenile |
| 5 | - | 2019. 10. 10. | Unknown | Corpse | |
| 6* | - | 2019. 11. 13. | Unknown | Corpse |
Table 2
The result of stable isotope analysis in survey area
| Spring | Summer | Fall | Winter | |||||
|---|---|---|---|---|---|---|---|---|
| Stable isotope | δ13C | δ15N | δ13C | δ15N | δ13C | δ15N | δ13C | δ15N |
| Bird | ||||||||
| Larus crassirostris | - | - | –22.51 | 17.14 | - | - | - | - |
| - | - | –19.49 | 15.68 | - | - | - | - | |
| - | - | –18.89 | 16.80 | - | - | - | - | |
| - | - | –19.34 | 15.55 | - | - | - | - | |
| - | - | –19.01 | 16.67 | - | - | - | - | |
| Average | δ13C, –19.85; δ15N, 16.37 | |||||||
| Mammalia | ||||||||
| Rattus norvegicus (Norway rat) |
- | - | - | - | –14.95 | 29.60 | - | - |
| - | - | - | - | - | - | –13.08 | 29.50 | |
| –16.67 | 29.60 | - | - | - | - | - | - | |
| - | - | - | - | - | - | –13.42 | 26.62 | |
| - | - | - | - | - | - | –11.5 | 32.65 | |
| Average | δ13C, -13.92; δ15N, 29.59 | |||||||
| Plantae | ||||||||
| Miscanthus sinensis | - | - | - | - | –26.87 | 21.33 | - | - |
| Eleusine indica | - | - | - | - | –13.79 | 34.03 | - | - |
| Albizia julibrissin | - | - | - | - | –28.42 | 18.62 | - | - |
| Average | δ13C, –23.03; δ15N, 24.66 | |||||||
| Insecta | ||||||||
| Isopoda sp. | - | - | - | - | –13.61 | 25.33 | - | - |
| Dermaptera sp. | - | - | - | - | –14.48 | 29.05 | - | - |
| Orthoptera sp. | - | - | - | - | –12.77 | 28.55 | - | - |
| Average | δ13C, –13.62; δ15N, 27.64 | |||||||