Introduction
Despite occupying less than one-fifth of the Earth’s terrestrial area (Dinerstein et al., 2017), tropical forests are crucial for public health, climate change mitigation, biodiversity, and the global economy, sequestering 10 million metric tons of carbon dioxide each year (Cook-Patton et al., 2020; Harris et al., 2021), homing 63% of the global terrestrial vertebrate species population (Pillay et al., 2022), and providing ecosystem services valued at 4.7 trillion USD per year (Costanza et al., 1997). However, humans have been decimating and fragmenting tropical forests for short-term economic gains, thus releasing carbon stocks into the atmosphere (Harris et al., 2021), leaving populations susceptible to the deleterious effects of reduced gene flow (Bacles et al., 2006), and increasing the costs of carbon stability (Fuss et al., 2021). Intact tropical forest fragments share edges with deforested lands or disturbed vegetation, and this accelerates their deterioration (Ingle, 2003).
In forest fragments, seed dispersal plays a critical role in supporting community structure and diversity by maintaining genetic connectivity across fragmented landscapes (Arteaga et al., 2006; Bacles et al., 2006). It influences future vegetation assemblages, rehabilitates degraded areas, and helps prevent further fragmentation (Gonzales et al., 2009; Ingle, 2003; McDonnell & Stiles, 1983). Since seed dispersal relies on abiotic and biotic agents, such as wind, water, and frugivorous vertebrates, determining the dominant dispersal agent can predict the direction of succession and vegetation assemblages (Janzen, 1983).
Anemochory (wind dispersal) and hydrochory (water dispersal) are secondary dispersal modes (Soomers et al., 2013) and can remobilize seeds that have been previously dispersed by other mechanisms (Hyslop & Trowsdale, 2012). Anemochory is the dominant dispersal mode in areas with strong winds (Howe & Smallwood, 1982). Some plants adapt specifically to anemochory; wind-dispersed fruits and seeds are usually winged, small, or flattened (Jongejans & Telenius, 2001). Meanwhile, hydrochory is crucial in sites near water bodies or sites in contact with water during storm surges or floods (Cappers, 1993). Unlike anemochory, hydrochory does not require seeds to have special adaptations (Hyslop & Trowsdale, 2012). It increases species richness, influences seed bank structuring, and dictates the vegetation composition in floodplains (Hayashi et al., 2008) and riparian areas (Jansson et al., 2005).
Meanwhile, animal dispersal assists tropical and subtropical forests by influencing vegetation composition, rehabilitating degraded lands, and supporting the large-scale exchange of genetic information between populations (Baltzinger et al., 2019; Houngbégnon et al., 2023; Howe & Smallwood, 1982; Wunderle, 1997). Animal dispersal helps plant populations escape the effects of climate change by facilitating their migration to suitable environments (Corlett, 2011; Lee et al., 2022). In a given submontane forest in the Philippines, animal dispersal is the mode of seed dispersal for 80% of all tree species (Hamann & Curio, 1999). Among animal dispersers, frugivorous birds and bats disperse more species and seeds at farther distances than their non-flying counterparts (Galindo-González et al., 2000). They relieve intraspecific competition among plants and facilitate the colonization of new areas (Howe & Smallwood, 1982).
Bats play a vital role in seed dispersal and reforestation in the neotropics (Arteaga et al., 2006). Fruit bats disperse seeds by (1) dropping fruits mid-flight to feeding roosts, (2) ejecting seeds in pellets, (3) ejecting seeds in feces, and (4) shaking off seeds stuck to their fur (Shanahan et al., 2001). Bats can forage in more open habitats than birds as the latter are more susceptible to predators during the day (Whittaker & Jones, 1994). However, seed dispersal studies in the Philippines that compared bird and bat dispersal revealed that birds dispersed more species and seeds than bats (Gonzales et al., 2009; Ingle, 2003). Birds are generalists; they fly faster, fly farther away, and have longer gut retention times than bats (Gonzales et al., 2009; Whittaker & Jones, 1994). In Luzon, Philippines, large avian frugivores are among the top seed dispersers in rainforests (Corlett, 2017). In areas depleted of large-bodied avian frugivores, small fruit (<14 mm) plant species dominate the seed rain and direct the subsequent woody succession, resulting in the establishment of a low-diversity “pioneer desert” (Martinez-Garza & Howe, 2003; Corlett, 2011).
The Caliraya Watershed has a peculiar geographic history. In 1939, the US Army Corps of Engineers constructed the Caliraya Dam that flooded portions of the Caliraya Watershed, resulting in the creation of the now-standing Caliraya Lake (National Power Corporation, 2018). New seed dispersal patterns may arise from the introduction of water. Water inundation can disperse seeds along the shore of the watershed and in the islets formed within the lake.
We aimed to achieve the following objectives in the Caliraya Watershed area: (1) determine the most important seed dispersal agents, (2) determine the floral species dispersed, and (3) investigate how the physical characteristics of the study site influence the seed dispersal patterns. Similar to other seed dispersal studies in the Philippines, we expected bird dispersal to be the dominant dispersal mechanism. As the Caliraya Watershed is characterized by strong winds, we expected wind to play a major role in dispersal due to the presence of wind-dispersed tree species. Since Caliraya Lake is a man-made lake, we assumed that water would play a minimal role in seed dispersal because of the vegetation present in the area. Lastly, we expected a positive relationship between seed density and distance from the shore, as the inland portions of the forest are not significantly affected by strong winds or flooding, both of which can affect seed distribution.
Materials and Methods
Description of study site
The study was conducted in a 500,000 m2 privately-owned, lowland secondary forest (14°19'11″ "N, 121°34' 4.5"″ E) at an elevation of 827 m in the northeast area of the Caliraya Watershed in Laguna, Philippines (Fig. 1). Within the 500,000 m2 study site, portions of a secondary forest were converted into agricultural and residential land. The area experiences Type II climate under the Corona Climate System of Classification, characterized by no dry season, maximum rainfall from December to February, and minimum rainfall from March to May (National Power Corporation, 2018).
Remote sensing
We assessed the current and historical land use in the study area using remote spatial analysis. We (1) delineated the area and perimeter of the study site, (2) pinpointed the location of the sampling sites, and (3) reviewed previous literature that classified the land cover in the Caliraya-Lumot watershed area. The study site was classified according to the following landscape features: agricultural, built-up, forest, non-forest vegetation (brushlands, grasslands, and shrublands), and water.
Floral survey
We surveyed the floral composition of the study site in a 12,000 m2 plot. Six points, spaced 20 m apart, were demarcated along the shore of the study area. Using these initial points as shore markers, subsequent points were placed 20, 40, 60, and 80 m from the shore, resulting in a total of 30 sampling points (Fig. 1). The coordinates of each sampling point were recorded.
From the last week of March to the first week of April 2022, we conducted a floral survey to determine the vegetation assemblage in the study plot. For each tree and shrub whose crowns were within a 5-m radius of each seed trap pair, we (1) measured the diameter at breast height, (2) estimated the height, and (3) identified the tree/shrub or collected voucher specimens if the identification was unknown. Within and beyond the study plot, we collected fruits and seeds that served as references for seed identification. Species were identified in situ using various keys and as many resources as possible, including Dayan et al. (2006), Merrill (1912), and Pelser et al. (2011). Voucher specimens were collected and deposited in the Jose Vera Santos Memorial Herbarium (PUH) at the Institute of Biology, UP Diliman. We measured the forest stand height using Lorey’s mean height, which uses the height of individual trees in proportion to their basal area (Nakai et al., 2010).
Bird and bat survey
We surveyed the composition of the frugivorous bird community within the study site using point counts and opportunistic sampling. Five points spaced approximately 100 m apart were used as sampling points; the first point was farther inward and higher than the fifth point, which was close to the shore (Fig. 1). We surveyed birds from May 31, 2022, to June 06, 2022. The survey was conducted for 400 minutes. For the point counts, five sampling points were observed for 10 minutes each. The point counts were performed eight times during the survey period. All surveys were conducted within an hour of sunrise (0530H-0630H) or an hour before sunset (1730H-1830H). As the quality of the recorded bird calls and songs was poor, only visual observations were considered for the analysis. At each point, we (1) identified the bird species, (2) recorded bird species abundance, and (3) photographed the birds whenever possible. The birds were identified based on Allen (2020) and validated by Dr. Carmela Española of the Institute of Biology, University of the Philippines Diliman.
We surveyed the composition of the frugivorous bat community using mist-netting at two different points within the study site from May 30, 2022, to June 05, 2022, to avoid surveying during the full moon. The survey was conducted for 168 hours (6 nets×4 hours×7 nights). We set up mist nets at 1,800 hours; we monitored the nets at intervals of 30 minutes. Each captured bat was subjected to the following before release: (1) measurement of standard bat morphometrics, (2) sex and age determinations, and (3) marking for identification. The bats were identified based on the key described by Ingle and Heaney (1992). Species accumulation curves were generated after both surveys to assess the sampling completeness.
Seed rain measurement
Wind, bird, and bat seed dispersal were investigated by collecting seed rain using seed traps. These seed traps, based on the design by Gonzales et al. (2009) and Ingle (2003), captured seed rain from wind, birds, and bats. Each seed trap was 1 m2 and consisted of a shallow cone of a satin-like green cloth with a mesh size of 0.1 mm. The cloth was attached to a circular bamboo frame using Velcro tabs and supported by four 1-m-high bamboo legs. The bottom of the cloth was tied to a stake on the ground to hold the trap in place because of the strong winds in the area.
The seed traps ran from the last week of March 2022 to the first week of June 2022, for a total of 11 weeks. At all sampling points in the 12,000 m2 study plot, a seed trap pair was placed; overall, there were 60 seed traps (2 seed traps×30 sampling points). One was randomly assigned as the “day” trap and the other served as the “night” trap. A brown or black cloth was used to cover the day trap during the nighttime and the night trap during the daytime. The covers were switched at dawn and dusk, depending on the time of sunrise and sunset, for all seed traps within 30 minutes.
At the end of each month, the contents of the seed traps were collected and air dried. Fruits and seeds in the traps were sorted from debris and transferred to airtight zipper storage bags. We examined and photographed the seeds under a dissecting microscope for identification and reference purposes. If the seeds collected from a seed trap pair were of the same species as a tree within a 5 m radius, these seeds were not included in the analysis for those seed traps to avoid counting gravity-dispersed seeds.
Based on morphological observations, data reported in various studies (Dayan et al., 2006; Esser, 2003; Hamann & Curio, 1999; Ko et al., 1998; Lee et al., 2017), and seed reference collections from floral sampling, we identified and categorized seeds into three dispersal modes: bat-dispersed, bird-dispersed, and wind-dispersed modes. Multiple unidentified diaspores and seeds were included in this analysis. We grouped them according to their morphological characteristics, assigned them to morphospecies, and categorized them according to their dispersal modes. The dispersal modes were identified by checking their morphological characteristics for adaptation to wind or animal dispersal and by cross-referencing previous literature. If the morphospecies could not be categorized by their morphological characteristics or by using data from previous literature, we checked their distribution in the seed traps: morphospecies found exclusively in the day traps were deemed to have bird-dispersed seeds, morphospecies found exclusively in night traps were deemed to have bat-dispersed seeds, and morphospecies found in both traps were deemed wind-dispersed seeds.
Because the seed traps were raised from the ground, we assumed that non-volant animals had little to no contribution to the seed rain. Although we observed some civet feces filled with fig seeds a few meters away from the seed rain sampling site, the contributions of arboreal vertebrates are minimal compared to those of birds and bats (Ingle, 2003). During data processing, we observed that several seeds had successfully germinated 10 weeks after collection. We calculated the percentage germination by accounting for successful versus total seeds of the morphospecies in question in each batch.
Assessing seed predation and seed recovery efficiency
To assess the effects of seed predation on the seed traps, four white kidney beans Phaseolus vulgaris were placed in each of the traps 4 days before the end of the seed-trapping period in June 2022. We assumed that the unrecovered seeds were lost to predation. To assess the efficiency of recovering small seeds from the seed traps, random numbers (0-9; mean, 4) of Ficus nota seeds that were dyed brown were placed in the seed traps on the last day of the seed-trapping period in June 2022.
Drift litter sampling
We investigated water seed dispersal by collecting drift litter at the study site, based on the methodology of Cappers (1993) and Jansson et al. (2005). Drift litter was collected twice: at the start of the seed rain measurement in March and at the end of the seed rain measurement in June. Drift litter was collected from seven different points along the shore of Caliraya Lake, near the study site (Fig. 1). At each point, we selected the portion with the most drift litter and collected drift litter that covered a 20×30 cm area. The drift litter samples were stored in large airtight zipper storage bags filled with fresh water and washed through a set of sieves with mesh sizes of 2 mm, 1 mm, and 0.5 mm. Sorted seeds were examined under a dissecting microscope and photographs were taken for reference. Seeds were identified according to morphological observations, data reported in various studies (Dayan et al., 2006; Esser, 2003; Hamann & Curio, 1999; Ko et al., 1998; Lee et al., 2017), and seed reference collections from floral sampling.
Data analyses
We used one-way ANOVA to determine whether seed density (seeds/trap) and species number (species/trap) significantly differed among bird-, bat-, and wind-dispersal modes. As the dependent variables were not normally distributed, seed density was transformed to ln (seed density+0.01). We used nonmetric multidimensional scaling (NMDS) to explore the relationship between floral species composition and distance from the shore and the relationship between avian species composition and distance from the shore. NMDS handles a large number of zeroes (absence data) in the species composition matrices. NMDS analyses were performed using the vegan package version 2.4-3 in R (Oksanen et al., 2017). Furthermore, we used Chi-square tests to confirm whether the numbers of bat- and bird-dispersed seeds collected in the seed traps were higher than those expected by chance. Fisher’s exact test was used to analyze bat- and bird-dispersed seeds with <5 samples in seed traps.
We used Morisita’s index of dispersion (I) to determine the distribution pattern of seed rain: uniform, random, or clumped. Separate values were determined for seed rain in the day (Iday), night (Inight), and day+night (Itotal). Morisita’s index of dispersion values is 0 for uniform, 1 for random, and >1 for clumped distribution (Morisita, 1962).
We used principal component analysis (PCA) followed by generalized linear model (GLM) regression analysis with Poisson errors to identify the factors that best accounted for seed density and species abundance patterns. The following factors were included in the PCA: distance between seed trap and shore, tree count (TN), tree basal area (TBA), fleshy-fruited tree count (FTN), fleshy-fruited tree basal area (FTBA), and occurrence of conspecific plants within a 5-m radius. Based on the PCA results, we performed GLM regression analysis with Poisson or quasi-Poisson errors and adopted the model with the lowest Akaike Information Criterion for overall seed dispersal and each dispersal type.
Results
Land cover classification
The land cover maps generated by Abino (2012) and Nuque (2018) illustrate a substantial landscape change in the Caliraya-Lumot watershed (Fig. 2). From 2012 to 2018, the proportions of agricultural and built-up areas decreased, whereas those of non-forest vegetation (brushlands, grassland, and shrublands) areas increased. Forest area accounted for 6.8% (728 ha) of the 10,734 ha Caliraya-Lumot watershed area analyzed in the Nuque (2018) land cover map (Table 1).
Floral community composition
In the 12,000 m2 study plot, we observed 905 individual trees belonging to 97 species. The forest stand height was 7.91 m high. The study plot was dominated by Macaranga tanarius (15.0%), Utania philippinensis (6.6%), and Ficus fistulosa (4.9%). The distance from the shore played a role in determining floral species composition (Fig. 3). To some degree, the shore sampling points were dissimilar to non-shore sampling points.
Bird and bat community compositions
We recorded 636 individual birds belonging to 35 species from 22 families (Table 2). We observed 5.1% (35 of 683 species) of all known bird species in the Philippines (Allen et al., 2020). A total of 51.4% (18 out of 35) of the observed bird species were known to have fruits and seeds in their diet: 11 omnivores, 6 frugivores, and 1 granivore. Large avian frugivores, such as the Luzon hornbill Penelopides manillae, Philippine cuckoo dove Macropygia tenuirostris, and White-eared brown dove Phapitreron leucotis, were also observed within the area. Frugivorous birds dominated the study site at 41.4% (263 of 636 individuals), followed by carnivores/insectivores (38.2%), omnivores (18.9%), and granivores (1.6%) (Fig. 4).
Elevation and distance from the shore played a non-significant role in determining the avian species composition (Fig. 5). We observed the highest species richness at Point E (closest to the lake), with 14 species, and the lowest at Point C, with 10 species (Table 3). We observed the highest abundance at Point A (176 individuals) and the lowest abundance at Point E (101 individuals) (Table 2). Shore sampling points (Points D and E) showed a slight bias toward water-associated bird species: Barred Rail Hypotaenidia torquata, Striated heron Butorides striata, and White-throated kingfisher Halcyon gularis were only observed near the shore.
We captured 28 frugivorous bats belonging to three species. The Lesser short-nosed fruit bat Cynopterus brachyotis accounted for 75% of the total capture, whereas Geoffroy’s rousettes Rousettus amplexicaudatus, 14%, and the Greater musky fruit bat Ptenochirus jagori, 11%.
Water-dispersed seeds
The collected drift litter contained a total of 339 seeds, representing seven morphospecies (Table 4). Ficus spp. seeds accounted for more than half of the seeds collected from the drift litter: F. nota accounted for 39.2% (133 of 339 seeds), and F. fistulosa accounted for 14.2% (48 of 339 seeds). Apart from the wind-dispersed C. formosum, all morphospecies collected from the drift litter had animal-dispersed seeds. None of the seeds were adapted to water dispersal. Water acted as a secondary disperser, dispersing seeds that were initially dispersed by other dispersal agents.
Overall composition of the seed rain
We collected 25,623 diaspores and seeds in the seed traps within the 11-week sampling period (March to June 2022). After eliminating the seeds that were of the same species as some trees within a 5-m radius from the seed traps where they were collected, we obtained 14,090 diaspores and seeds for data analysis (herein, we will refer to the dispersal units as seeds).
The seeds represented 52 species and morphospecies:19 were bird-dispersed, 13 were bat-dispersed, 9 were both bat- and bird-dispersed, and 12 were wind-dispersed seeds. Bird- (n=166 seeds/trap) and bat-dispersed seeds (n=145 seeds/trap) had significantly higher seed densities (no. seeds per seed trap) than wind-dispersed (n=79 seeds/trap) seeds (Fig. 6; One-way ANOVA: F2,87=16.21, P<0.0001). Birds (n=3.7 species/trap) and bats (n=3.9 species/trap) dispersed seeds belonging to a significantly higher number of species than did the wind (n=0.2 species/trap) (One-way ANOVA: F2,87=16.67, P<0.0001).
Chi-square (X2=4,914.4, P<0.0001) and Fisher’s tests (P<0.0001) revealed that animal-dispersed seeds were significantly biased toward either bat or bird dispersal (Fig. 7). Bird-biased seeds were small; bat-biased seeds were large, green, and had firm pericarps. Seed rain was distributed among the traps in clumps (Itotal=1.30). Bird-dispersed seeds (Ibird=1.25) were distributed in clumps, whereas bat- and wind-dispersed seeds were randomly distributed (Ibat=1.01; Iwind=1.00).
The first two principal components (PC1 and PC2) accounted for 80% of the variance in seed density and species abundance, based on the physical characteristics of the forests (Fig. 8). Tree count and fleshy fruit tree count were weighted heavily in PC1. Distance from shore, tree basal area, and fleshy fruit tree basal area were weighted heavily in PC2.
In the GLMs, none of the factors significantly influenced the overall seed density distribution of total, bat-dispersed, and wind-dispersed seeds (Table 5). The fleshy fruit tree count, tree count, fleshy fruit tree basal area, and tree basal area significantly influenced the seed density distribution of bird-dispersed seeds. All factors, except distance from shore and occurrence of conspecific seed source, significantly affected the morphospecies number in the collected seeds (Table 5). The tree basal area significantly affected the morphospecies number of bird-dispersed seeds. Fleshy-fruited tree basal area and tree basal area significantly affected the morphospecies number of bat-dispersed seeds. None of the factors included in the GLM significantly affected the morphospecies number of wind-dispersed seeds.
Apart from wind-dispersed seeds, tree basal area significantly affected the number of morphospecies of total, bird-dispersed, and bat-dispersed seeds. The distance from shore and occurrence of conspecific seed sources did not significantly affect seed density or morphospecies number.
Seed predation and seed recovery efficiency
In the seed predation experiment, approximately 90.8% (218 of 240) of the kidney bean seeds placed in the traps were recovered intact in 96.7% (58 of 60) of the seed traps. In the seed recovery experiment, 77.6% (198 of 255) of the F. nota seeds placed in the traps were recovered in 94.2% (49 of 52) of seed traps. The effects of seed predation and the possibility of undercounting seeds were assumed to be relatively small.
Germination of dispersed seeds
Ten weeks after seed rain collection, we found that several seeds had successfully germinated (Table 6). We calculated the percent germination by calculating the successful versus total seeds of the morphospecies in question in each batch. Unknown C, an animal-dispersed morphospecies that most likely belonged to Macaranga or Homalanthus, had the highest percent germination at 82.1% (32 of 39 seeds). All the germinated seeds were animal dispersed.
Discussion
Summary of wildlife biodiversity surveys
The dominance of early successional species, such as M. tanarius, U. philippinensis, and F. fistulosa, and the significant dominance of bat- and bird-dispersed species in the seed rain experiment indicate that the forest is in its early-to-mid successional stage, especially in the inland areas (Whittaker et al., 1989; Yassir et al., 2010). Bat- and bird-dispersed M. tanarius and F. fistulosa (Peh & Chong, 2003; Whittaker & Jones, 1994) were early successional species on Mt. Krakatau after its eruption in 1883 (Whittaker & Jones, 1994). The exclusively bat-dispersed U. philippinensis (which previously belonged to the genus Fagraea) is an early successional species in fire-razed grasslands in Indonesia (Yassir et al., 2010). Additionally, all three species are fire-resistant (Whittaker et al., 1989; Yassir et al., 2010) and may have developed this feature to ensure their survival in fire-prone watersheds.
The floral composition of the sampling points 0 m away from the shore was quite dissimilar from the compositions of the other plots. Dillenia philippinensis, Hopea acuminata, and Urostigma spp. were exclusively located at sampling points 0 m from the shore. The sampling points 20, 40, 60, and 80 m away shared a degree of similarity. As the shore sampling points are more visible to law enforcement agencies, they are better protected from illegal logging, charcoal harvesting, and slash-and-burn farming, whereas non-shore sampling points are not. In the absence of the threat of anthropogenic disturbances, economically valuable trees commonly traded for their timber, such as Dipterocarpus hasseltii, Shorea assamica var. philippinensis, and Shorea contorta, are left untouched at the shore sampling points.
Among frugivorous birds, bulbuls comprised 63.9% (251 out of 393 individuals), with 103 Hypsipetes philippinus individuals and 148 Pycnonotus goiavier individuals. Bulbuls consume small-sized fruits and seeds. Bulbuls were one of the first post-eruption colonizers at Mt. Krakatau (Whittaker & Jones, 1994) and are one of the most important seed dispersers in deforested tropical forests in Asia (Corlett, 2017). Both H. philippinus and P. goiavier have also been reported in other Philippine seed dispersal studies (Gonzales et al., 2009; Ingle, 2003). Apart from bulbuls, large avian frugivores such as Macropygia tenuirostris, Penelopides manillae, and Phapitreron leucotis were also observed. Large avian frugivores provide seed dispersal services that are essential for forest regeneration dynamics and floral species richness (Española et al., 2013). Local extinction of these frugivores would deprive approximately 60% of late-successional floral species in Philippine submontane rainforests of their dispersal agents (Hamann & Curio, 1999).
As the bird survey relied on point counts and opportunistic sampling, we obtained only a snapshot of the bird community assemblage at the study site. Balatibat (2008) conducted a wildlife survey in the Caliraya Watershed and recorded 16 more frugivorous bird species, including Bolbopsittacus lunulatus and the vulnerable Gallicolumba luzonica.
The avian compositions at the five different sampling points were quite similar, with only certain species differentiating the sampling points from each other. Eurystomus orientalis and Spilopelia chinensis were exclusively observed at Point A. Meanwhile, water-associated birds such as Ardea cinerea, Butorides striata, and Halcyon gularis were only found near the shore at Points D and E.
The total number of frugivorous bat species captured during the study was 20.0% (3 of 15 species) of all known fruit bat species on Luzon Island. Among netted fruit bats, the most common species was C. brachyotis, accounting for 75% of the total capture. C. brachyotis and P. jagori can be found in both forests and highly disturbed habitats (Duya et al., 2020). The presence of C. brachyotis and P. jagori could be attributed to the high abundance of Ficus species, since they are the dispersers for this species (Hamann & Curio, 1999; Lim et al., 2018).
Given that sampling was only performed for a total of 168 net hours in the span of seven nights and considering the species accumulation curve, we did not sample all the bat species in the area, such as the Arcuate horseshoe bat Rhinolophus arcuatus recorded previously by Balatibat (2008). Despite having captured only three species of frugivorous bats, the overall effect of low bat diversity on seed dispersal services was minimal because the observed bats are generalists (Duya et al., 2020; Hamann & Curio, 1999). However, an increase in bat species richness can increase seed species richness by promoting the dispersal of a wide variety of fruits (Aguilar-Garavito et al., 2014).
Animal dispersal was higher than wind and water dispersal
Water dispersal plays a minimal role in influencing seed dispersal patterns in the Caliraya Watershed. The formation of the 83-year young oligotrophic Caliraya Lake did not drastically change the vegetation in the area to warrant the adaptation to hydrochory. As demonstrated in the drift litter experiment, hydrochory did not exclusively disperse any species and only served as a secondary dispersal agent. The seeds collected from the drift litter experiment did not have any specific adaptations to water dispersals, such as buoyancy, large surface area, or water-repellant surface (Cappers, 1993); instead, they were dispersed by sticking to debris and flotsam (Hyslop & Trowsdale, 2012). Therefore, hydrochory plays a minimal role in seed dispersal patterns in the Caliraya Watershed.
F. fistulosa and F. nota seeds accounted for 53.4% (181 of 338 seeds) of all water-dispersed seeds. Ficus species are usually dispersed along water bodies, especially riverbanks. For example, F. carica seeds are dispersed in large quantities by the River Rhine in the Netherlands (Cappers, 1993), whereas female F. squamosa trees mainly produce figs during the rainy season as an adaptation to water dispersal (Pothasin et al., 2016).
Despite its minimal role in seed dispersal, hydrochory is important in maintaining the genetic connectivity of forests within the watershed, especially in areas separated by large distances of water that zoochory and anemochory cannot cross. In the Amazon basin, the Rio Branco River facilitates gene connectivity among all plant species, regardless of their mode of dispersal (Nazareno et al., 2021). Caliraya Lake does not act as a barrier, and local adaptation to hydrochory is unlikely. However, water-dispersed fruits and seeds, such as Barringtonia sp. and Cocos nucifera were observed outside the drift-litter experiment area, indicating the possibility of a shift in hydrochory patterns in the Caliraya Watershed.
As the Caliraya Watershed is characterized by strong winds, anemochory was expected to strongly influence the seed dispersal patterns. However, animal-dispersed seeds predominated over wind-dispersed seeds in terms of seed density and the number of morphospecies. Although anemochory dispersed significantly fewer seeds and species, it strongly influenced the seed dispersal patterns. Twelve different morphospecies were exclusively wind-dispersed. Most of the wind-dispersed seeds, C. formosum, D. hasseltii, and Unknown AF, were small and specifically adapted for anemochory. C. formosum, a pioneer species in lowland tropical forests (Ahmad Fitri et al., 2022), is the most abundant wind-dispersed species. Although there was a high density of C. formosum seeds in some of the seed traps, we did not find C. formosum seedlings at the study site. Because wind-dispersed seeds are usually small and the study site was filled with forest litter, their establishment success rate is low (Ingle, 2003).
The bias toward animal dispersal can be attributed to the floral community in the study plot. More than half (489 of 905) of the individual trees surveyed had fleshy fruits that attracted animal dispersers (Howe & Smallwood, 1982). From the floral survey, we observed that Macaranga and Ficus trees were the most abundant and frequently dispersed by birds and bats. Moreover, the number of both bat- and bird-dispersed species was twice more than the wind-dispersed species.
Several seeds had successfully germinated after collection. All these seeds belonged to animal-dispersed morphospecies, further emphasizing the importance of animal dispersal in restoring degraded habitats (Wunderle, 1997). Previous studies have reported that ingesting and subsequently excreting seeds increase the germination percentage and rate (Nakamoto et al., 2007; Utzurrum & Heidemann, 1991). In a previous study (Enriquez, 2019, unpublished), we reported that the passage of F. nota seeds through the guts of C. brachyotis and P. jagori did not negatively affect the germination percentage and rate.
Birds and bats disperse different species of trees
This study illustrates that frugivorous birds exclusively disperse more species than bats, albeit not at a statistically significant level. Birds also exclusively dispersed Melicope triphylla, the fourth most abundant tree species in the study plot. M. triphylla is an early successional species and was one of the first colonizers on Taal Volcano Island after the 1911 eruption (Gates, 1917). Because of the frequent rainfall in the Caliraya Watershed, the competition between bat and bird dispersal rates may not be pronounced due to the abundant fruit supply (Gonzales et al., 2009). However, the slight differences between bat and bird dispersal rates should not be ignored. Since there is a wider variety of fruits available for birds than for bats, frugivorous birds have more opportunities to disperse seeds. Furthermore, the unique feeding behavior of bats, where they have separate fruit source and feeding areas, affects their seed dispersal patterns (Corlett, 2017). Because the study site is mainly dominated by M. tanarius, F. fistulosa, and U. philippinensis, which produce fruits that are attractive to bats (Hamann & Curio, 1999; Lee et al., 2017; Peh & Chong, 2003; Whittaker & Jones, 1994), bats may have frequented the study site to obtain fruits but not stayed long enough to disperse seeds through ejecta, pellets, or feces (Gonzales et al., 2009).
This study illustrates that bird and bat dispersal usually targets different fruits or seeds. Macaranga species were significantly biased toward bird dispersal, whereas Ficus species were significantly biased toward bat dispersal. As bats and birds usually target different fruits, the perceived competition is low. The separation of the seed dispersal services of bats and birds (Mello et al., 2011) implies that both are necessary for enhancing seed dispersal patterns and forest regeneration in the Caliraya Watershed.
Physical characteristics of the forest affected bird dispersal
The distance from the shore did not affect the dispersal patterns of bat-, bird-, and wind-dispersed seeds. Instead, the tree basal area and fleshy fruit tree basal area dictated most of the dispersal patterns. They influenced the density distribution of bird-dispersed seeds and the morphospecies number of bird- and bat-dispersed seeds. Gonzales et al. (2009) reported that the number and total basal area of fleshy fruit trees mainly dictated the dispersal patterns in the Subic Watershed Forest Reserve. It has been previously documented that fleshy fruit trees are the dispersal foci in semi-deciduous forests (Clark et al., 2004).
Bird-dispersed seeds were distributed in clumps, whereas bat-dispersed seeds were distributed randomly, according to Morisita’s index of dispersion. As mentioned earlier, bats might forage in the study plot and consume the fruits in feeding roosts in other locations, which explains the random distribution and weak association of the physical characteristics of the study plot and their seed dispersal patterns. On the other hand, birds do not exhibit this feeding behavior and linger much longer in the study plot and are more susceptible to plot characteristics influencing their seed dispersal patterns. Bird-dispersed seeds are usually deposited in clumps under the fruiting trees where they feed (Clark et al., 2004).
Implications to tropical forest restoration
Although direct comparisons cannot be made between Abino's (2012) and Nuque (2018) Caliraya–Lumot Watershed land cover maps because they included different land areas and used different models or algorithms to classify land cover, both maps illustrate that forests only account for approximately 6-7% of the total land cover. Forests only account for 7,280,000 m2 of the 163,190,000 m2 combined total land cover of the Caliraya–Lumot Watershed, a far cry from the 96,040,000 m2 permanent forest reserve designated by Presidential Proclamation No. 573 (National Power Corporation, 2018). The severe deforestation in these watersheds can be mainly attributed to anthropogenic activities such as illegal logging, squatting, and land conversion (Jose & Cardenas, 2010).
With natural and anthropogenic disturbances inhibiting the growth of large plants, non-forest vegetation (brushlands, grasslands, and shrublands) now accounts for 51% (54,520,000 m2) of the total land cover of the Caliraya–Lumot Watershed. The dominance of non-forest vegetation illustrates that most areas in the watershed are in the early stages of secondary succession in the aftermath of continuous disturbances (Brown et al., 1917). Similar fire disturbances (through volcanic eruptions) on Mt. Krakatau (Whittaker et al., 1989), Mt. Pinatubo (Marler & Moral, 2011), and Mt. Taal (Brown et al., 1917; Gates, 1917) revealed that brushes, grasses, and shrubs were the earliest colonizers of razed vegetation. Islets within the lake and lakeside areas were dominated by local pioneer fern species (possibly Sticherus sp.) that completely covered some islets and hindered the growth of other species. Intentional fires sparked by locals alter the disturbance regimes of the watershed and expose it to invasive plants (Miller et al., 2021). If a site is continuously subjected to repeated fire disturbances, the floral composition of the study site is highly likely to be homogenized (Popradit et al., 2015). Furthermore, climax tree species, such as dipterocarps, are felled by locals who conduct illegal timber harvesting. Economically valuable dipterocarps only remain along the shoreline as they are more visible to law enforcement personnel.
Determining seed dispersal patterns can help reforestation efforts at deforested sites. Our results could provide insights for future forest restoration efforts in the Caliraya Watershed or in patches with similar floral compositions, characteristics, and landscapes. Since both bats and birds dispersed more seeds and species, we believe that protecting existing and planting pioneer fleshy fruit trees in reforestation zones will greatly expedite seed dispersal and forest regeneration. Macaranga and Ficus species are among the best candidates, as they attract a variety of frugivores and have strong colonization abilities. Furthermore, Ficus species are also key resources for supporting other frugivorous and insectivorous populations in tropical forests, as they provide habitat and a year-round food source. Meanwhile, reforestation schemes should focus on planting and enriching mid-successional species (Hamann and Curio, 1999). As the seed establishment of wind-dispersed species is poor, clearing the forest will improve restoration efforts (Hardwick et al., 1997). Because seed dispersal is a two-way mutualistic process, it is necessary to enact and enforce laws that will protect the flora and fauna. As the forests in the Caliraya Watershed are largely fragmented and threatened by anthropogenic activities, conserving the remaining patches is necessary to facilitate connectivity among large habitats. Forest restoration efforts should utilize a three-way approach: (1) ensuring the presence of fleshy fruit trees in restoration zones, (2) assisting the establishment of mid-successional and wind-dispersed trees, and (3) strict law enforcement and intensification of conservation efforts for both flora and faunal diversity.
Acknowledgments
We would like to sincerely thank Tammy Africa for generously allowing us to conduct our study in their property, Dr. Carmela Española, Ramon Bandong, and Abigail Garrino for help in identifying uncommon species, and Dr. Nina Ingle and Dr. Regielene Gonzales for the guidance throughout the whole study. We would also like to acknowledge the UP Diliman College of Science and the UP Institute of Biology for providing permits to conduct field experiments in our study sites. This study was funded by the Department of Science and Technology – Higher Education Institute through the Accelerated Science and Technology Human Resource Development Program.
Author Contributions
GPLE conceptualized the study, conducted fieldwork and laboratory work, wrote the earlier drafts of the manuscript, performed data analyses, and provided final approval of the article. LJVR conceptualized the study, contributed to fieldwork, performed data analyses, and prepared the final manuscript.
References
National Power Corporation (2018, Retrieved Feb 17, 2022) Caliraya-Lumot watershed from https://www.napocor.gov.ph/
Nuque, E (Cartographer) (2018) Caliraya Watershed [Webmap] Retrieved from: https://www.arcgis.com/home/item.html?id=3ce15e051a5349bdbfd0b60935b9a131
, , , , , , et al. (2022, Retrieved Aug 18, 2022) vegan: community ecology package. R package version 2.6-2 from https://CRAN.R-project.org/package=vegan
, , (2011, Retrieved Aug 18, 2022) 2011 onwards. Co's digital flora of the Philippines from www.philippineplants.org
Figures and Tables
Fig. 1
Map showing the arrangement of the different sampling points in the study site (14°19'11" N, 121°34' 4.5" E). Pink circles represent the floral survey sampling points and the seed rain seed trap pairs, blue triangles represent the bird survey sampling points, yellow pentagons represent the bat survey sampling points, and green stars represent the drift litter sampling points. The map was generated using ArcGIS software by Esri.
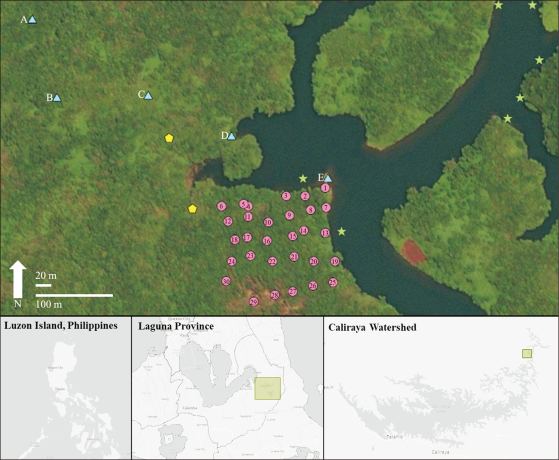
Fig. 2
Land cover maps of the Caliraya-Lumot watershed area depicting the land use change from 2012 to 2018. Maps were acquired from Nuque (2018) and Abino (2012).
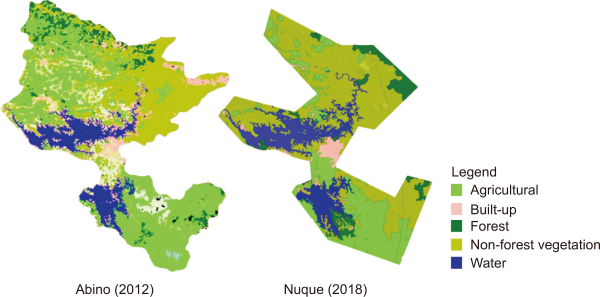
Fig. 3
Nonmetric multidimensional scaling (NMDS) plot showing the relationship of the floral composition and distance from shore in Caliraya, Laguna for March 2022. Each color indicates the distance from shore: 0 m=green, 20 m=orange, 40 m=purple, 60 m=yellow, and 80 m=black line. NMDS stress value was 0.12.
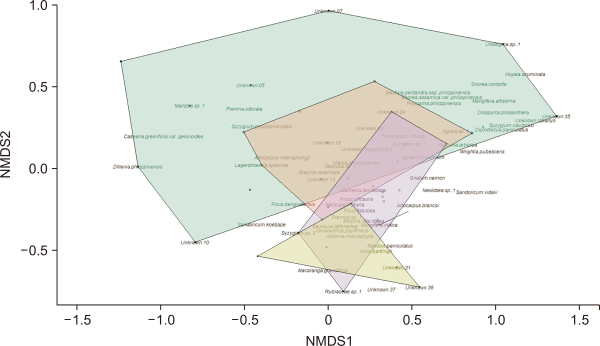
Fig. 4
Nonmetric multidimensional scaling (NMDS) plot showing bird species composition similarity across the five sampling points in Caliraya, Laguna for May-June 2022. Each color indicates a sampling point: A=green, B=purple, C=orange, D=yellow, and E=blue. NMDS stress value was 0.13.
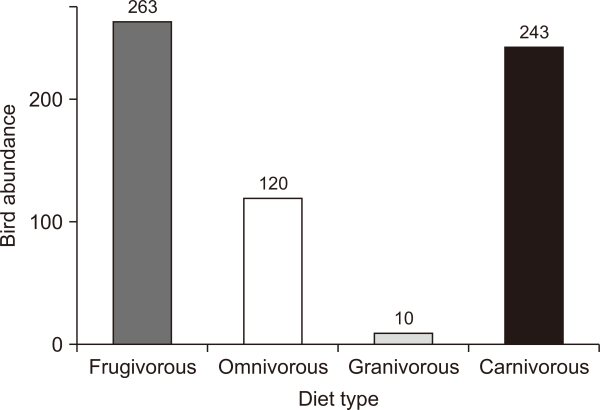
Fig. 5
Abundance of bird species based on their diet. Birds with fruit- and seed-based diet make up for 62% of total recorded bird counts.
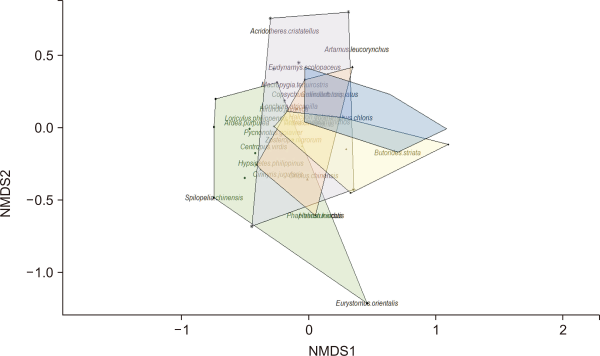
Fig. 6
Mosaic plot showing the bias of the twenty most abundant animal-dispersed seeds towards either bat (light bars) or bird dispersal (dark bars). Longer bars of one dispersal agent represent higher bias towards either dispersal agent in terms of the number of seeds dispersed. Meanwhile, wider bars represent greater abundance of the seeds from a species collected in the seed rain collection.
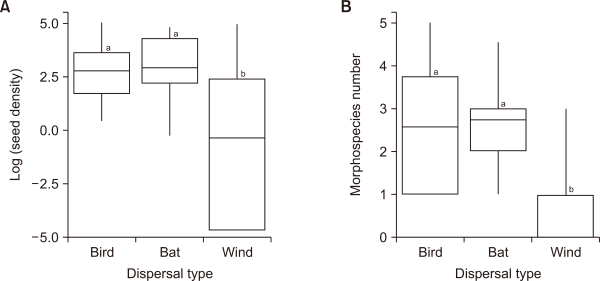
Fig. 7
Differences in (A) seed density (no. of seeds/seed trap) and (B) morphospecies number (no. of seed species/trap) based on dispersal type. Shown are the means and 95% confidence intervals.
Fig. 8
Principal component analysis of the different physical factors that influence seed dispersal patterns: distance from shore, tree count, tree basal area, fleshy-fruited tree count, fleshy-fruited tree basal area, and occurrence of conspecific plants within a 5-m radius.
Table 1
Difference in land cover (%) of agricultural, built-up, forest, non-forest vegetation, and water areas in the Caliraya-Lumot watershed area from 2012 to 2018
| Land classification | 2012 land cover (%) |
2018 land cover (%) |
Change |
|---|---|---|---|
| Agricultural | 30.91 | 23.10 | –7.82 |
| Built-up | 17.45 | 2.70 | –14.74 |
| Forest | 6.17 | 6.78 | +0.62 |
| Non-forest vegetation |
29.15 | 50.79 | +21.64 |
| Water | 16.32 | 16.62 | +0.30 |
Table 2
Species of birds recorded in the study area through point counts and opportunistic sampling
| Family | Scientific name | Common name | Seed diet |
|---|---|---|---|
| Accipitridae | Haliastur indus | Brahminy kite | No |
| Alcedinidae | Ceyx cyanopectus | Northern-indigo banded kingfisher | No |
| Halcyon gularis | White-throated kingfisher | No | |
| Todiramphus chloris | Collared kingfisher | No | |
| Ardeidae | Ardea alba | Great egret | No |
| Ardea cinerea | Grey heron | No | |
| Ardea purpurea | Purple heron | No | |
| Bubulcus ibis | Cattle egret | No | |
| Butorides striata | Striated heron | No | |
| Egretta garzetta | Little egret | No | |
| Artamidae | Artamus leucorynchus | White-breasted woodswallow | No |
| Bucerotidae | Penelopides manillae | Luzon hornbill | Yes* |
| Cisticolidae | Orthotomus derbianus | Grey-backed tailorbird | No |
| Columbidae | Chalcophaps indica | Grey-capped emerald dove | Yes* |
| Macropygia tenuirostris | Philippine cuckoo dove | Yes* | |
| Phapitreron leucotis | White-eared brown dove | Yes* | |
| Spilopelia chinensis | Eastern spotted dove | Yes† | |
| Coraciidae | Eurystomus orientalis | Oriental dollarbird | No |
| Corvidae | Corvus macrorhynchos | Large-billed crow | Yes‡ |
| Cuculidae | Centropus virdis | Philippine coucal | No |
| Dasylophus superciliosus | Red-crested malkoha | No | |
| Eudynamys scolopaceus | Asian koel | Yes† | |
| Estrildidae | Lonchura atricapilla | Chestnut munia | Yes§ |
| Hirundinidae | Hirundo javanica | House swallow | No |
| Meropidae | Merops americanus | Rufous crowned bee-eater | No |
| Monarchidae | Hypothymis azurea | Black-naped monarch | Yes† |
| Muscicapidae | Copsychus mindanensis | Philippine magpie-robin | Yes† |
| Nectariniidae | Cinnyris jugularis | Olive-backed sunbird | Yes† |
| Oriolidae | Oriolus chinensis | Black-naped oriole | Yes† |
| Psittacidae | Loriculus philippensis | Philippine hanging parrot | Yes* |
| Pycnonotidae | Hypsipetes philippinus | Philippine bulbul | Yes∥ |
| Pycnonotus goiavier | Yellow-vented bulbul | Yes‡ | |
| Rallidae | Hypotaenidia torquata | Barred rail | Yes† |
| Sturnidae | Acridotheres cristatellus | Crested myna | Yes§ |
| Zosteropidae | Zosterops nigrorum | Yellowish white-eye | Yes∥ |
Classification of fruit diet was mainly based on *Española et al. (2013), †Corlett (2017), ‡Whittaker and Jones (1994), §Snow (1981), and ∥Ingle (2003).
Table 3
Species abundance and richness of birds observed in Caliraya, Laguna in June 2022, and the elevation and distance from the lake of each sampling point
| Point | Species richness | Species abundance | Elevation (m) | Distance (m) |
|---|---|---|---|---|
| A | 12 | 176 | 837 | 0 |
| B | 13 | 125 | 832 | 100 |
| C | 10 | 129 | 829 | 200 |
| D | 13 | 105 | 827 | 300 |
| E | 14 | 101 | 818 | 400 |
Table 4
Morphospecies of water-dispersed seeds collected from two sampling instances in seven different points in the Caliraya Lake, Laguna from March to June 2022
| Morphospecies | Seed density (no. of seeds) |
Dispersal syndrome |
|---|---|---|
| Cratoxylum formosum | 63 | Wind |
| Ficus fistulosa | 48 | Bat, Bird |
| Ficus nota | 133 | Bat, Bird |
| Macaranga cumingii | 26 | Bat, Bird |
| Macaranga tanarius | 28 | Bat, Bird |
| Omalanthus fastuosus | 2 | Bird |
| Unknown C | 39 | Bat, Bird |
Table 5
Values reported are t-values (seed density) and z-values (morphospecies no.) from the generalized linear models
| Factors | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Distance from shore |
Tree count | Fleshy-fruited tree count |
Tree basal area | Fleshy-fruited tree basal area |
Conspecific plants within a 5-m radius | |
| Seed density | ||||||
| Total | - | - | –1.01 | - | 1.40 | - |
| Bird | 0.40 | –2.55* | 2.28* | 3.46** | –2.82** | 0.25 |
| Bat | - | - | 1.17 | - | 0.81 | - |
| Wind | - | - | –1.32 | - | 1.13 | - |
| Species | ||||||
| Total | - | –2.26* | 2.09* | 2.74** | –2.16* | - |
| Bird | - | –1.24 | 0.58 | 2.05* | –1.52 | - |
| Bat | - | –1.50 | 1.60 | 2.49* | –1.98* | - |
| Wind | - | - | 1.18 | - | –0.08 | - |
Table 6
Percent germination of morphospecies that germinated during sample processing
| Morphospecies | Percent germination (%) | Dispersal syndrome |
|---|---|---|
| Breynia vitis-idaea | 5.00 | Bat |
| Macaranga cumingii | 11.76 | Bat, Bird |
| Macaranga sp. | 1.01 | Bat, Bird |
| Utania philippinensis | 5.97 | Bat |
| Unknown C | 82.05 | Bat, Bird |
| Unknown E | 12.90 | Bat, Bird |
| Unknown AR | 7.65 | Bat |