Introduction
Obtaining accurate information on the evolutionary relationships between organisms is important for solving many biological questions. Based on this information, revealing the phylogenetic relationships based on the results of experiments and observations is complicated. Morphological phylogenetic studies have been performed based on species selection, dominant traits, and polygenic inheritance (Friesen et al., 1996; Thomas et al., 2004a). Therefore, molecular analysis, such as DNA sequencing, is not only a useful method for logical inferences to support or disprove previous hypotheses about phylogenetic relationships among species but also an appropriate method to provide a further rational hypothesis in explaining the evolutionary history of species (Baker, 1969; 2006; Baker et al., 2007). Particularly, the mitochondrial DNA (mtDNA) sequence of each species is advantageous in studying the intraspecies differences in genetic sequences because it is less prone to the high possibilities of mutation and DNA recombination and exhibits maternal inheritance (Ericson et al., 2003; Gyllensten et al., 1991; Verkuil et al., 2010). Additionally, mtDNA is also widely used in molecular analysis to draw species branch diagrams (Wenink et al., 1996). The Kentish Plover (Charadrius alexandrinus Linnaeus 1758) belongs to the Charadriidae family. Three suborders are present in Charadriiformes, (Björklund, 1994; Pereira & Baker, 2010), and Charadriidae is included in Charadrii. Charadriiformes is an effective clade for phylogenetic research because it shows not only various specifications but also distinctive traits in evolutionary history (Fain & Houde, 2007; Sibley et al., 1988). Additionally, Charadriiformes is useful for modeling, which is ideal for investigating the macroevolution of waterbirds (Thomas et al., 2004a). Furthermore, molecular analytical methods have developed new interpretations and provided various perspectives on the phylogenetic diversification of Charadriiformes (Baker et al., 2007; Paton et al., 2003). For instance, until the early 21st century, the Snowy Plover (Charadrius nivosus Cassin 1858) was regarded as a subspecies of the Kentish Plovers (C. a. nivosus); however, molecular phylogenetic analysis has revealed several differences between them (Küpper et al., 2007; 2009). Although these molecular phylogenetic studies separated two different species (Kentish and Snowy Plover), the exact phylogenetic relevance of C. alexandrines and C. nivosus pertaining to the unexplained parts, such as the adult appearance, ecological position, and haplotype comparisons need to be studied (Küpper et al., 2007; 2009; Thomas et al., 2007).
In this study, we reviewed to: (1) examine the representative ecological characteristics of the Kentish and Snowy Plovers; (2) compare the morphological and genetic differences between C. alexandrinus and C. nivosus; and (3) describe the morphological and genetic differences among their subspecies.
The Kentish Plover (Charadrius alexandrinus)
Unlike the name suggests, the Kentish Plovers do not breed only in Kent or across the UK. They are widely distributed in areas, such as Europe, North Africa, Asia, and Middle to South America (Kerr et al., 2007; Mcdonald et al., 2022). Its population size is estimated to be approximately 300,000-460,000 worldwide (Tayefeh et al., 2021). On the Korean Peninsula, almost 100 pairs of breeding individuals and 1,000-10,000 individuals of migrant groups have been regularly found (Cotgreave & Harvey, 1992; Kim et al., 1994). A gradual decrease in the overall population size of this species has been observed (Brazil, 2009).
Morphological features and ecology
The body size of the Kentish Plovers is 15-17 cm. They have longer legs and thinner beaks than those of other species in the genus Charadrius, such as the Ringed Plover (C. hiaticula) or the Semipalmated Plover (C. semipalmatus). Their bodies have a grayish-brown dorsal region and a white ventral region. Particularly, the dark neck-band pattern on the side of the chest is completely disconnected.
The Kentish Plovers generally breed in inland lakes, sandy beaches, or salty water; however, they are not typically observed in fresh waters such as the upper to middle rivers. They dig sandy grounds to make nests and lay three to five eggs during the breeding season. The breeding populations of the Kentish Plover in tropical countries are settled or distributed within narrow ranges (del Hoyo et al., 1992; Lessells, 1984); however, most inland or coastal populations in the North Sea are completely migratory and widely move from north to south in winters (Mcdonald et al., 2022).
Classification system
The scientific name of the Kentish Plover is Charadrius alexandrinus Linnaeus, 1758 (Küpper et al., 2007; 2009). Several representative subspecies of Charadrius alexandrinus (del Hoyo et al., 1992) exist such as C. a. alexandrinus Linnaeus, 1758 from Europe to East Asia, C. a. dealbatus from Southeast China to Indonesia (MacKinnon & Phillipps, 2000; Yang et al., 2010), and C. a. seebohmi from Southeast India to Sri Lanka (Grimmett et al., 2016; Hartert & Jackson, 1915).
The Snowy Plover
Morphological characteristics and ecology
The Western Snowy Plover is a small black-billed plover. This species has a light brown or grayish dorsal region, white or buff-colored ventral region, and dark-colored shoulders and head (Powell & Collier, 2000). Furthermore, they have a black line behind their eyes and a white forehead. Compared to the Kentish Plovers, snowy Plovers have shorter legs and the heads of the males lack reddish-brown crowns during the breeding period (Brindock & Colwell, 2011; Lafferty et al., 2006; Neuman et al., 2004; Ruhlen et al., 2006). A slightly developed eye mask is found in the Indian and Sri Lankan Snowy Plovers. Particularly, compared to other plover species, dark brown or black legs are the distinctive features of the Snowy Plovers (Grimmett et al., 2016; Page et al., 1995).
The populations of Snowy Plovers in the Pacific coastal area breed near tidal areas and on all west coasts, peninsulas, bays, and estuaries of the mainland United States (Nur et al., 1999). The reported habitat ranges of Snowy Plovers range from Damon Point and Washington, USA to Bahia Magdelena and Baja California, Mexico (Pearson et al., 2007). However, some exceptions exist depending on their geographic distribution. When measured at similar elevations, the Kentish Plovers from Al Wathba are lighter than the Snowy Plovers from Ceuta.
Classification system
The scientific name of the Snowy Plover is Charadrius nivosus Cassin 1858. Pluvierneigeux is another common name. Representative subspecies exist such as C. n. nivosus Cassin, 1858, C. n. tenuirostris from the USA to Mexico, and C. n. occidentalis from Peru and Chile (D’Urban et al., 2020). Classification of these subspecies was performed based on recent studies on mtDNA and microsatellite analyses (Funk et al., 2007; Verkuil et al., 2010).
Genetic Relationships between the Kentish and Snowy Plovers
Genetic diversification
Phylogenetic studies using CHD (chromo-helicase-DNA-binding) sequence and microsatellite loci have demonstrated consistent genetic differences between the Kentish and Snowy Plovers (Küpper et al., 2007; 2009). This shows that marine geographic barriers prevented gene flow between the Eurasian and American populations. Additionally, this genetic diversification suggests that gene flow did not occur over a long period (Küpper et al., 2007; Price, 2007). mtDNA sequences and CHD-Z genotypes showed a 6% difference between the Kentish and Snowy Plovers, whereas they showed a 2% difference (Lloyd, 2008) between the Kentish and White-fronted Plovers (Charadrius marginatus). This suggests that the divergence between the Snowy and Kentish Plovers occurred earlier than that between the Kentish and White-fronted Plovers (Küpper et al., 2009). Furthermore, the Kentish Plover showed significantly higher genetic diversity than the White-fronted and Snowy Plovers. Considering the variables influencing the selection of gene markers, the diversity of mitochondrial sequences is higher in Kentish Plovers than that in the Snowy Plovers (Küpper et al., 2007; 2009).
Genetic divergence among the subspecies of Snowy Plovers in America did not show any genetic differences (Funk et al., 2007) because the genetic differences among geographically separated populations of the same species (C. n. nivosus) were impossible to compare. Subsequently, mitochondrial haplotype analysis indicated that the effect of the geographical differences was not related to C. a. nivosus and C. a. alexandrinus because the haplotypes of the populations in Spain and the UAE showed both samples having the same haplotype. Notably, the differences between the American Snowy Plover subspecies as described in Funk et al. (2007) were much smaller than those between the intercontinental groups presented here (Küpper et al., 2007; 2009).
Phenotypic differences
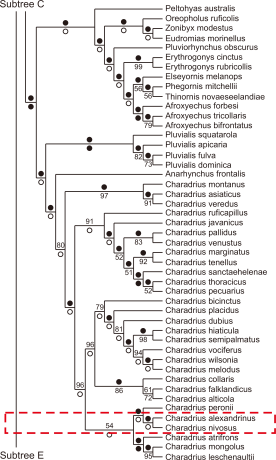
The wings and tarsus of Kentish Plovers are significantly larger than those of snowy Plovers (Hayman et al., 1986). Body mass is also higher in the Kentish Plovers than that in Snowy Plovers. Additionally, the differences in down feather patterns separate the Kentish and Snowy plovers because differences in patterns play important roles in the phylogenetic classification of diverse shorebird species (Jehl, 1968; Paton et al., 2002). Interestingly, considering that the patterns of down feathers are used for camouflage, they are largely influenced by geographical characteristics. Moreover, various subspecies have not been classified and are together considered to be only one species widely distributed in various locations because further diversity across subspecies is observed in adult birds. (Oberholser, 1922). Finally, the cladistic maps of the Kentish (Charadrius alexandrinus) and Snowy Plovers (Charadrius nivosus) drawn based on the above contexts are shown in Figs. 1 and 2 (Livezey, 2010).
Conclusion
Until a recent study on the phylogenetic tree of the Kentish Plovers, several previous studies have been conducted not only on the phylogenetic classification of Charadriiformes (Björklund, 1994; Hewitt, 1996; Joseph et al., 1999; Livezey, 2010; Thomas et al., 2004a) but also on the different perspectives of classifying various species of Charadrius using fossil records, genetic analysis, and morphological or behavioral characteristics (Funk et al., 2007; Küpper et al., 2007; 2009).
The results of the analysis using mtDNA and CHD genes showed that the Snowy Plovers, which were the focus of this study, separated from the Kentish Plovers earlier than the White-fronted Plovers. Despite geographic differences, genetic diversity between populations within a single species has not been found (Küpper et al., 2009). However, in interspecific comparisons, these genetic differences lead to phenotypic differences among the species. The Snowy Plovers have shorter legs than the Kentish Plovers. The Snowy Plovers also have brown or black legs, whereas the Kentish Plovers do not. Additionally, male Kentish Plovers have reddish-brown crowns on their foreheads during the breeding season, whereas male Snowy Plovers do not. The Kentish Plovers generally have a heavier body mass, larger wings, and a longer tarsus than the Snowy Plovers (Cotgreave & Harvey, 1992). The phylogenetic relationship between the two species has been confirmed through behavioral analysis. In addition to the molecular phylogenetic evidence of the differences between the Kentish and Snowy Plovers, studies on other trait differences are needed. Therefore, we suggest that studies on phylogeny be conducted not only to compare species (C. nivosus nivosus and C. a. alexandrinus) but also to compare the subspecies of the Kentish (C. a. alexandrinus, C. a. dealbatus, and C. a. seebohmi) or Snowy Plovers (C. n. nivosus, C. n. tenuirostris, and C. n. occidentalis). For instance, C. a. dealbatus and C. a. seebohmi are classified as the subspecies of C. alexandrinus. However, this needs to be supported by experimental evidence. We expect that conducting studies on these aspects will be useful to explain the complicated process of divergence of C. alexandrinus (Thomas et al., 2004b).
Author Contributions
Conception, design, and manuscript drafting and writing: Woo Yuel Kim. Sample collection and manuscript writing: Gun-hwa Kang, Dong Yun Lee. Manuscript drafting and literature review: Ha Cheol Sung.
Figures
Fig. 1
Higher-order groups of Charadriiformes in the present study. Majority rule consensus (MRC) tree for subordinal and family-group nodes. All nodes shown are conserved at 100% in an MRC tree. Dashes indicate nodes that are either monotypic or analysed as familial exemplars; bootstrap percentages (○, <50%; ●, 100%); decay (Bremer) indices are given below selected branches. Adapted from Livezey. Phylogenetics of modern shorebirds (Charadriiformes) based on phenotypic evidence: analysis and discussion; 2010.
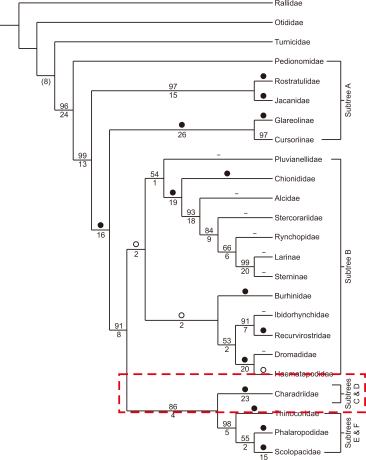
Fig. 2
Subtree D. Majority rule consensus (MRC) tree for species of Eudromianinae and Charadriinae in the present study. Percentages for branches in the MRC are given above the branches (●, 100%), and bootstrap percentages are given below branches (○, <50%; ●, 100%). Adapted from Livezey. Phylogenetics of modern shorebirds (Charadriiformes) based on phenotypic evidence: analysis and discussion; 2010.