Introduction
The leopard (Panthera pardus) is the most widespread large cat in the world (Nowell & Jackson, 1996), and occupies a wide range of habitats, including rainforests and desert mountains in temperate regions and the fringes of urban areas (Kitchener, 1991). Despite having the widest distribution, leopards are often assumed to be of low conservation priority among large felids because of the limited information available across their range, except for African studies (Balme et al., 2012). Habitat loss, prey depletion, retaliatory killing, and poaching for trade have caused a decline in leopard populations (Nowak, 1999; WPSI, 2006), which are currently categorized as near-threatened by the International Union for Conservation of Nature (Henschel et al., 2008).
Leopards show plasticity in their behavior as conditions change and are adaptive to dwell near human habitations (Seidensticker, 1990) which often results in direct conflict with people mainly in the form of over-livestock predation (Athreya, 2006). Hence, leopards are prone to retaliatory killing or poaching. Thus, reliable data on the population densities of leopards are needed to develop sound conservation and management strategies (Thapa et al., 2014) and to identify priorities in the allocation of limited resources (Karanth, 2003). Leopards are difficult to monitor because of their nocturnal, cryptic, and elusive behavior; wide home ranges; and low population densities (Cederlund et al., 1998). Traditionally, pugmarks have been used to estimate the abundance of large carnivores (Panwar, 1979), but these techniques have been criticized and found to be less reliable (Karanth et al., 2003). With the development of automatically triggered camera traps, they have been widely used to estimate the abundance of species through individual identification based on their unique coat pattern (i.e., tiger (Panthera tigris), Karanth, 1995; leopard (P.P) (Gray & Prum, 2012)).
In India, leopards are listed in Schedule I of the Indian Wildlife (Protection) Act, 1972, and are under the highest level of protection, but reliable information on the abundance of leopards is still scarce compared to other sympatric carnivores, such as tigers (Jhala et al., 2011; Panda et al., 2022a). The present study was part of a long-term study conducted in the Ranthambhore Tiger Reserve (RTR), India (Singh et al., 2022). We estimated the population density of leopards in a semi-arid habitat of the RTR in western India, which is known to have a relatively high tiger density (Karanth et al., 2004), and provided baseline data for the conservation of this elusive species.
Materials and Methods
Study area
The RTR (25° 54ʹ 26° 12ʹ N, 76° 22ʹ 76° 39ʹ E), situated in the semi-arid region of Rajasthan (Fig. 1), western India, is a transition zone between desert and peninsular India (Rodgers & Panwar, 1988) and is dominated by Northern tropical, dry, deciduous, and thorny forests (Champion & Seth, 1968). The TR is surrounded by 332 villages within 5 km of the reserve boundary, with more than 150,000 people and livestock (Bagchi et al., 2003). People in the region around the reserve chop grass and trees, in addition to grazing livestock. This region receives 800 mm of precipitation each year with temperatures ranging from 2°C in January to 47°C in May (Singh et al., 2013). The RTR has a subtropical dry climate with four distinct seasons: summer (March to June), monsoon (July to August), post-monsoon (September to October), and winter (November to February). The terrain, with 76% of the area categorized as highly undulating, has an elevation gradient ranging from 200 to 500 m (Singh et al., 2021). Besides the leopard (Panthera pardus), other large predators in the RTR include the tiger (Panthera tigris) and striped hyena (Hyaena hyaena) (Singh et al., 2020). The study area also supports five species of wild ungulates: chital (Axis axis), sambar (Rusa unicolor), nilgai (Boselaphus tragocamelus), chinkara (Gazella gazelle), wild pig (Sus scrofa), sloth bears (Melursus ursinus), common langur (Semnopithecus entellus), and peafowl (Pavo cristatus) (Panda et al., 2022b).
Sampling design
For camera trapping, we created a grid system based on distribution maps generated by indirect sign surveys (i.e., tracks and scat). Cameras were built and placed near the most frequently used carnivorous pathways to ensure that every individual in the research area could be identified (Wilson & Delahay, 2001). The sampling region was divided into 1x1 km grids, and the cameras were positioned sequentially across the grid. At any given time, we used 60 remotely triggered cameras: 13 active infrared systems (TrailMaster TM1550; Goodson and Associates, Inc., USA) and 47 digital passive infrared systems. The passive infrared systems comprised 13 Wildview systems (Wildview, USA), 9 Stealth Cam units (Stealth Cam, USA), and 25 Moultrie systems (Moultrie Game Feeders, USA). Each Trail Master unit was connected to a fully automatic 35 mm camera (Canon A1 Mini DX; Canon USA, Inc., USA) with automatic flashes. Considering that different cameras have varying detection zones, we set the cameras in a way ensuring there is enough time to identify an animal and take full-frame photographs of the subject, including three shots constantly taken at short intervals. White flashes could be seen up to 30-35 m away from both the active and passive cameras.
Each sampling grid was composed of a single camera placed 6-7 m away from the road to capture each flank of a passing leopard (Karanth & Nichols, 1998). In “survey design 4”, the research area was divided into 3 consecutive spatially separated blocks and systematically sampled in a phased manner (Karanth & Nichols, 2002; Fig. 1). Two camera units were used in the third block during the sampling period; thus, we had 58 sampling stations in that block. Sampling took place between the 5th of December 2010 and the 20th of February 2011, with a 25-day sampling interval in each block. We took 2-3-day breaks after each trapping session (25 days) to download the images from the digital cameras and change the cameras in the following block. The camera traps were operated 24 hours a day, 7 days a week. Because of the good road network, all trapping stations were monitored on alternate days throughout the sampling period. All the covered camera-trapping sites had a minimum convex polygon (Fig. 1) of 233 km2.
Leopard density
Individual leopards were identified from photographs obtained using camera traps by visually examining the markings on the pelages of hind limbs, forelimbs, and forequarters (Kalle et al., 2011). Individually identifiable leopards in the photographs were allocated unique identification numbers and the trap site, sample period, date, and time of capture were documented. We created a striped leopard capture history in spatial explicit capture-recapture (SECR) data format for analysis, taking into account a continuous 75-day sampling occasion (1-to-25-day sessions in block A, 26-to-50-day sessions in block B, and 51-to-75-day sessions in block C) (Singh et al., 2014).
We followed the SECR approach to obtain maximum likelihood density estimates for leopards using camera trapping data (Efford, 2011).
The likelihood SECR models were subjected to the R packages SECR and DENSITY 5.0 (Efford 2010; Efford et al., 2009; www.otago.ac.nz/density). The detection probability of each individual was modeled using the spatial detection function (Efford, 2004) and was explained by 2 parameters (one-night detection probability at the center of an individual’s home range, [go], and a function of the scale of animal movements, [σ]; Efford, 2004). We chose a half-normal detection algorithm because it appears to be acceptable for mark-recapture data from large carnivores and is the most commonly used. By integrating the Poisson distribution of the home range centers and adding a buffer of 10,000 m around the trapping grids, we evaluated the log-likelihood function (Zimmermann et al., 2013).
Results
Based on the total sampling effort of 4,450 active trap days (2 camera traps were stolen) from 178 active trapping stations between December 2010 and February 2011, we obtained 53 photographs of leopards, of which 46 were suitable for individual identification (25 right flanks and 21 left flanks). The right flank photo capture was taken for further analysis, as it involved a larger number of individuals and captures. In total, 18 individual leopards (7 males, 8 females, and 3 unknown sexes) were identified based on the right flank. The statistical test for population closure in the CAPTURE program (Otis et al., 1978; Rexstad & Burnham, 1991) supported the assumption that the sampled population was closed during the sampling period (z=–0.403, P=0.340). Additionally, leopard density was 8.8 (standard error=2.8) individuals/100 km2.
Discussion
The leopard density was estimated to be 8.8 per 100 km2 in our study area which was similar to that of the Sariska Tiger Reserve (STR) (8.0 per 100 km2; Mondal et al., 2012a), which has a similar habitat and is adjacent to our study region. Both study areas are situated in the semi-arid region of Western India, and the vegetation is composed of tropical open thorny forests with highly undulating topography and high human influence. As. The RTR maintains a high prey density of medium-to-large-sized wild prey species (96.5 per km2; Bagchi et al., 2003) which supports the high density of carnivores, as it is believed that carnivore density is positively correlated with prey biomass (Carbone & Gittleman, 2002; Karanth et al., 2004). Leopards in India prefer small-to-medium-sized prey (chitals, wild pigs, and langur), generally weighing less than 50 kg (Harihar et al., 2011; Johnsingh, 1992; Karanth & Sunquist, 1995; Sehgal et al., 2022). Among the prey species, chitals were the most frequently consumed prey item by leopards in the Indian subcontinent (Andheria et al., 2007; Ramesh et al., 2009) and leopard density was positively correlated (R2=0.6) with chital density (Fig. 2). According to Bagchi et al. (2003), chital (31 animal/km2) was the most abundant wild prey in the RTR, followed by common langur (21.75 animal/km2), sambar (17.15 animal/km2), and wild pig (9.77 animal/km2). Furthermore, the leopard density derived from camera traps in the present study was close to the estimates obtained from the prey biomass-carnivore relationship (Carbone & Gittleman, 2002). Similarly, relatively higher leopard density has also been reported in other protected areas (PA) of tropical dry deciduous forests, that is, the Pench Tiger Reserve (9/100 km2, Majumdar, 2011); and Mudumalai Tiger Reserve (13.7/100 km2; Kalle et al., 2011). Both PAs had higher prey densities (Majumdar et al., 2012). However, the population density estimates of leopards in the PAs of moist deciduous forests of Rajaji National Park were estimated to be 2.07 to 9.72 leopards/km2 (Harihar et al., 2011) (Table 1). However, it has been speculated that the fluctuation in the density of leopard populations in the area may be a function of poaching (Johnsingh & Negi, 2003) or a response to the recovery of the tiger population (Harihar et al., 2011). The estimated leopard density in the lowland forest of Manas National Park (MNP) in the Eastern Himalayan Mountains is 3.4 /100 km2 (Borah et al., 2014). The MNP, which receives high rainfall (>2,800 mm) and has a closed canopy forest, has high primary productivity in the canopy relative to the ground; hence, a low density of prey and predators may be expected (Datta et al., 2008; Glanz, 1982). Variations in carnivore density between different ecoregions may be a function of vegetation type, prey availability, topography, rainfall patterns, large predator abundance, and hunting pressure (Chapron et al., 2008).
Other than prey density, additional factors, such as disease and interspecific competition, also contribute to variations in carnivore densities (Chauvenet et al., 2011; Harihar et al., 2011; Singh et al., 2015). With a simultaneous recovery of tiger populations, the density of leopards decreased from 9.76/100 km2 to 2.07/100 km2 in Rajaji National Park (Harihar et al., 2011) and 8.0/100 km2 to 3.3/100 km2 in STR (Mondal et al., 2012b). Our results showed that RTR had a lower tiger density (4.80 individuals/100 km2) than leopards, and a comparison of leopard density with tiger density across different regions suggested that leopard densities appear to be the response of tiger densities.
However, the tropical dry thorns and deciduous forests of India, including the RTR, have potential productive grassland habitats that are enriched with prey (Karanth et al., 2004a). Therefore, a higher leopard population density is expected in tropical dry-forested habitats. Chundawat et al. (1999) suggested that there are potential tropical dry forested habitats available in India, which extend to approximately 150,000 km2, and if such habitats can be protected, they can support a substantial number of large cats, such as tigers and leopards (Karanth et al., 2004b).
The results of this study provide baseline information on the leopard population in the RTR, and it is assumed that tropical dry forests provide suitable habitats for leopards and can support adequate leopard populations. Thus, it may be suggested that dry regions are potential areas of conservation importance for the maintenance of metapopulations. Thus, wildlife authorities must consider or include leopard populations when formulating conservation and management strategies.
Acknowledgments
We are grateful to the Director and Dean of the Wildlife Institute of India for their help. We are grateful to the Rajasthan Forest Department, as well as the reserve officials and field employees at the RTR for granting permission and allowing our work, as well as for their assistance. Special thanks go to the RTR’s nature guides and field helpers, M. S. Gurjar and S. Sharma, for their assistance. The Ministry of Environment and Forests, Government of India provided financial support through the Training Research and Academic Council.
References
, , , , , , et al. (2008, Retrieved Apr 13, 2023) In The IUCN Red List of Threatened Species v. 2015.2 Panthera pardus, from https://www.iucnredlist.org
WPSI (2006, Retrieved February 21, 2021) Skinning the cat: crime and politics of the big cat skin trade from http://www.indiaenvironmentportal.org.in/files/Skinning%20the%20Cat.pdf
Figures and Table
Fig. 1
Locations of camera traps used in Ranthambhore Tiger Reserve, western India, December 2010 to February 2011.
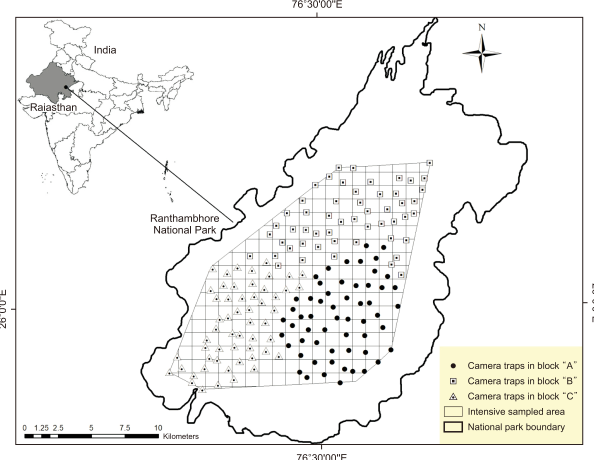
Fig. 2
Leopard density with relation to chital density in Indian-subcontinent (source data: leopard density (RCNP- Thapa, 2011; STR- Modal et al., 2012; RNP- Harihar et al., 2011; MTR- Kalle et al., 2011; PTR- Majumder et al., 2012), chital density (RCNP- Seidensticker, 1976; STR- Avinandan et al., 2008, RNP- Harihar et al., 2011; MTR and PTR- Majumder et al., 2012).
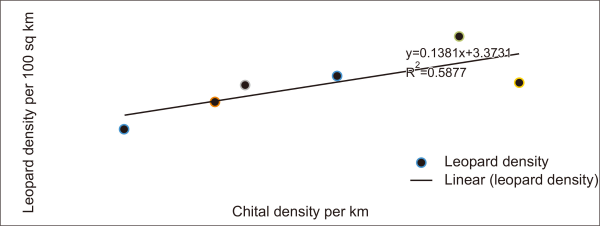
Table 1
Leopard and tiger density in Indian subcontinent with respect to habitat
| Study area | Habitat type | Leopard density | Tiger density | Referencea |
|---|---|---|---|---|
| Chilla National Park (2006) | Tropical moist deciduous | 9.72 | 3.31 | Harihar et al., 2011 |
| Chilla National Park (2010) | Tropical moist deciduous | 5.81 | 2.07 | Harihar et al., 2011 |
| Chitwan National Park | Tropical moist deciduous | 4.34 | 2.7c | Thapa, 2011 |
| Mudumalai Tiger Reserve | Tropical dry deciduous | 13.7 | 8.31 | Kalle et al., 2011 |
| Manas National Park | Tropical rain forest | 3.4 | 0.8b | Borah et al., 2013 |
| Pench Tiger Reserve | Tropical dry deciduous | 9 | 2.5b | Majumdar, 2011 |
| Ranthambhore Tiger Reserve | Tropical thorn forest | 8.8 | 4.72 | Present study |
| Sariska Tiger Reserve | Tropical thorn forest | 8.0 | Mondal et al., 2012 | |
| Sariska Tiger Reserve | Tropical thorn forest | 3.3 | Mondal et al., 2012 | |
| Satpura Tiger Reserve | Tropical dry deciduous | 5.9 | 1.57b | Edgaonkar et al., 2007 |