Introduction
Allometry is a study of the relationships between changes in a body part and its overall size (Levinton, 1988). Intraspecifically, morphological differences are not pronounced, because most traits are proportional to the body size (McCullough, 2015). However, certain traits increase in size faster than the overall body size. Thus, small individuals and large individuals can be proportionally different (McCullough, 2015). Among the traits, sexual structures are more variable than nonsexual structures, as recorded for most beetles (Romiti, 2015), such as Coleoptera: Lucanidae (Knell, 2004) and Coleoptera: Scarabaeoidae (Emlen and Nijhout, 1999).
The subfamily Dynastinae usually exhibits strong male dimorphism, especially exaggerated horns (McCullough, 2015). Such ornaments and weapons are signals that indicate the potential of a male to compete with other males or mates (Green, 1992). In addition, the presence of pronounced ornaments and weapons infer that the male is large and of a high-quality breed because positive allometric structures are expensive to produce and carry (Andersson, 1982; Kodric-Brown and Brown, 1984).
Lycomedes De Breme, 1844 is a genus that is distributed only in the Neotropical region, along the Andes from Colombia to Peru. Earlier, it was recognized as the same taxonomic group as Spodistes, but, at present, these two groups are considered distinct (Arrow, 1902). Resembling the other genus of Dynastinae, Lycomedes exhibits strong sexual dimorphism and intraspecific polymorphism. Although the subfamily Dynastinae is constituted of many polymorphic species, Dynastine beetles have received much less attention compared to other species like Lucanidae.
Among the ten species of Lycomedes, six are known in the Colombian Andes (Neita-Moreno & Ratcliffe, 2019), which is well known for its great biodiversity and the high levels of endemism (Myers, et al., 2000; Kattan, et al., 2004). In this study, the allometry of Lycomedes was analyzed for the first time in the literature.
Materials and Methods
Study design
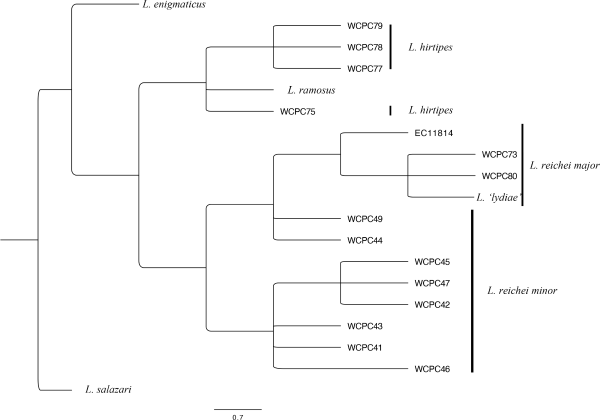
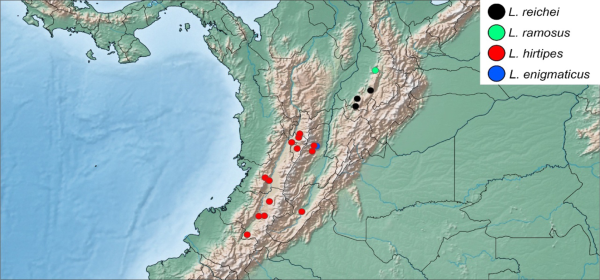
At first, we investigated the existence of a related species group among Colombian Lycomedes. Figure 1 shows the morphological phylogenetic results of this search. Additionally, the distribution data of the L. reichei species group is shown in Figure 2.
After basic research on the L. reichei species group, the allometry was analyzed within the group. Because L. reichei displayed the most dramatic allometry, further morphometric analysis was conducted using the data of L. reichei. Positive allometries were observed, and, finally, the clustering analysis confirmed that L. reichei could be separated into several groups based on morphological measurements.
Specimens for the study
A total of 19 specimens (Table 1) belonging to the following collections or institutes were studied. The male genitalia were extracted by using forceps. The parameters were glued on cardboard and pinned below the specimens.
Morphological characters and measurements
Specimens were examined under Nikon SMZ645 or photographed with Nikon D5200 (Tokyo, Japan) with a Tamron 90-mm Macro lens mounted on the Wemacro macro rail. Photos of each specimen were merged by using the Zerene Stacker program (Zerene Stacker, Richland, WA, USA). ImageJ (Caroline, Rasband, and Eliceiri, 2012) was used to measure each body part. The body length (BL) is the distance between the clypeus and the apex of the elytra. Elytra width (EW) and Elytra height (EH) refer to the largest distance of the elytron. Pronotum width (PW) is the largest width of the pronotum. Tooth height (TH) is measured by drawing an imaginary line A that halves the tooth, and a line B is drawn perpendicular to line A, contacting the apex of the clypeus, followed by measuring the length of line A from the intersecting point to the apex. Basal cephalic horn length (BCHL) is the distance from the apex of the clypeus to the anterior pronotal margin. Thoracic horn length (THL) is measured by drawing an imaginary line A that connects the anterior and posterior margin of the pronotum, and line B is drawn perpendicular to line A, where it contacts the apex of the thoracic horn, followed by measuring the length of line B from the intersecting point to the apex. Cephalic horn length (CHL) is the distance between the clypeus and the apex of the cephalic horn. Eye distance (ED) is the number of eye diameters that transverse the front (Fig. 3). Eye width (EW) is the longest distance of the eye from a dorsal view.
Data analysis
Statistical analysis was conducted with the software R (R Core Team, 2022). After each morphological characteristic was coded, phylogenic analysis was conducted with PHYLIP (Felsenstein, 2013). The distribution map of the species was prepared using SimpleMappr (Shorthouse, 2010).
Results
Lycomedes reichei species group
Among the Colombian Lycomedes, some species share more characteristics than others. The morphological phylogenetics results revealed that most of the Colombia Andean Lycomedes species, except L. salazari, belong to a single clade (Fig. 2). The results indicated the close relevance between L. hirtipes and L. reichei. Indeed, in minor males, the two species were nearly identical, except for a tooth on the base of the cephalic horn and the adeagus. In this study, four species were grouped into the species group. Among the group, L. reichei is the main subject that was analyzed owing to its dramatic allometries.
The L. reichei species group is constituted of the following four species: L. reichei, L. ramosus, L. enigmaticus, and L. hirtipes. This group is distributed along the Andes (Fig. 3) and characterized by the combination of the following morphological characteristics: males with cephalic horn bifurcated; ocular canthus transverse, tip with a short forward projection; perpendicular thoracic horn; prosternal process projected and hollowed; humeral and apical umbones projected; aedeagus symmetrical and elongated; a pair of keels somewhat prominent, forming depressions near margin, lower half slender.
Allometry of the L. reichei species group
Although most of the species in the L. reichei species group exhibit strong dimorphism, L. hirtipes is exceptional owing to the constant morphology of horns. Since the first description of L. hirtipes is based on a dozen specimens, no major males have been reported so far.
Among the population of L. reichei, two variations at the base of cephalic horns have been observed. A part of the population exhibits allometry at both cephalic and thoracic horns. In the majority of the male species, the base of the cephalic horn is elevated, forming a longitudinal, laterally flattened keel connecting the middle of the cephalic horn and frons, with the apical tip bifid (Fig. 4B). Medium males only have a moderately elevated keel with the apical tip (Fig. 4C). Hereafter, the above type of population is referred to as the “reichei form.” The other population exhibits pronounced allometry only at the thoracic horn (Fig. 4A).
This type of population is referred to as the “lydiae form.” Regardless of their type, minor males are nearly impossible to designate the form and have minute teeth at the base of the cephalic horn (Fig. 4E). Thus, minor males are morphologically similar to L. hirtipes (Figs. 4E-F).
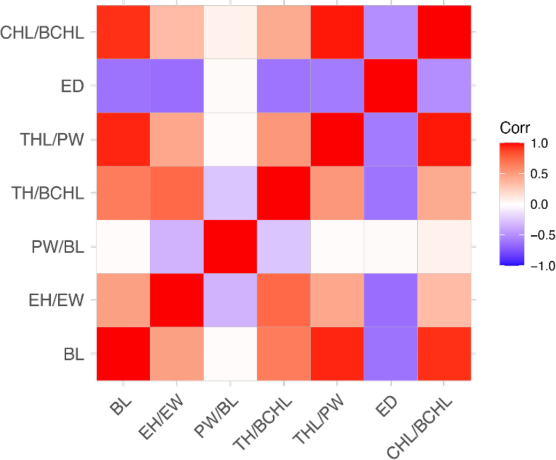
First, the ratio of various parts of the body was measured and analyzed. All analyzed traits, except ED, displayed a positive allometric relationship with respect to BL. Strikingly, most traits grew much faster in major males than in minor males. This acceleration of growth was observed in multiple ratios; EH/EL, TH/BCHL, THL/PW, and CHL/BCHL (Fig. 5). Differences between major males and minor males were found to be significant (p = 0.028, 0.034, 0.005, 0.01, respectively). Among the analyzed traits, EH and TH grew more exponentially than the other traits (correlation coefficient = 0.67, 0.68, respectively). However, the growth of EL and PW was steady. The ratios of EL and PW concerning BL were not significant between major males and minor males (p = 0.14, 0.57, respectively).
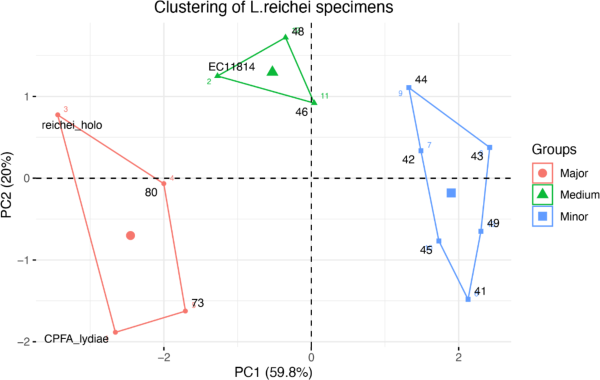
Principal component analysis revealed that most traits had a positive correlation with BL (Fig. 6). Especially, CHL/BCHL and THL/PW ratios were highly and positively correlated with BL and each other. In contrast, ED was negatively correlated with most of the traits. PW/BL, which was the least differentiated between major and minor males, displayed very weak or no correlation with the other traits. Interestingly, k-means clustering displayed three different clusters (Fig. 7). Specimens with major characteristics were clustered into group 1, and the ones with minor characteristics were clustered into group 3. Group 2, which is composed of EC11814, WCPC46, and WCPC48, can be regarded as the “medium males,” that exhibit intermediate characteristics. Indeed, WCPC46 and WCPC48 are relatively large individuals among the minor males, and EC11814 is the smallest individual within the major group.
Discussion
Relevant previous studies are mainly conducted with dung beetles (Emlen, 2000; Moczek, 2002; Emlen, 2005; Stanbrook, 2021), Lucanidae (Kawano, 2000; Knell et al., 2004; Knell, 2009; Iguchi, 2013), or horns of Dynastinae (Kawano, 1995; Álvarez, 2013). In the present study, an allometric analysis using various body parts was conducted for the first time in the subfamily Dynastinae, genus Lycomedes.
Allometry and ecology of the L. reichei species group
The L. reichei species group is distributed along the Andes (Fig. 3) (Milani, 2017; Neita-Moreno & Ratcliffe, 2019; Pardo-Locarno, 2020). L. reichei is originally reported as “Soccora” (Arrow, 1902), which is a misspelling of Soccoro. L. reichei can be found in Santander at an altitude of 1200–2200 m (Neita-Moreno & Ratcliffe, 2019; Pardo-Locarno, 2020). L. ramosus is reported at Cundinamarca and Boyacá (Pardo-Locarno, 2020). L. enigmaticus has been reported in Tolima at an altitude of 2406 m (Neita-Moreno & Ratcliffe, 2019). L. hirtipes is found at altitudes of 1,200 m to 2,100 m and in the following localities: Antioquia, Cauca, Huila, Quindio, Tolima, Risaralda, and Valle del Cauca (Pardo-Locarno, 2020).
Notably, the distribution of the group can be separated into two regions. L. hirtipes and L. enigmaticus, which are the species that lack structures at the base of cephalic horn are distributed in Cordillera Occidental and Cordillera Central. On the other hand, L. reichei and L. ramosus, cephalic horns that have structures, are distributed across Cordillera Oriental. Different vegetations of each cordillera may induce different morphological evolution. Interestingly, Colombia can be divided biogeographically into several provinces and districts, according to González-Orozco, 2021. Páramo province is a highly complex subregion in Colombia, which is divided into five districts and 11 sub-districts (González-Orozco, 2021). L. hirtipes is distributed across several districts, however, L. reichei and L. ramosus are restrictively distributed, only within one district.
In major males, the tooth at the base of the cephalic horns displays two morphologies. An acute and small tooth in the lydiae form, but a more robust, large, and longitudinally dilated structure was expressed in the reichei form. Variations of the tooth in the position and the morphology are evident in Lucanidae and some species of Dynastinae. However, in most cases, variations were detected in isolated populations. Thus, the variation in the tooth of L. reichei may represent the highly endemic nature of Andean species, which can be differentiated across the mountains. Although the distribution of L. reichei is very restricted, the endemism of the Andes may induce differentiation in a relatively small area, the Páramo region shows high speciation rates (Madriñán et al., 2013) due to the complex topography, wide altitudinal range, and the contrasts in soil mosaics (Etter & van Wyngaarden, 2000).
In tropical mountains like the Andes, zonation using bryophyte is useful (Gradstein et al., 1989). According to S. R. Gradstein et al., tropical mountains can be categorized into a few zones: The upper tropical submontane forest, 1200–2200 m, and the lower tropical montane forest, 2200–3000 m (S. R. Gradstein et al., 1989). More specifically, Homeier et al. described the Ecuadorian Andes into several vegetation types; evergreen montane forest, 1300–2100 m, and evergreen upper montane forest, 2100–2700 m (Homeier et al., 2008). Altitude contributes significantly to the diversity (González-Orozco, 2021). Furthermore, the “transition zone,” which is approximately 2100–2200 m, between two altitudinal belts may produce a variety of microclimates and unique vegetation. Indeed, the reported collecting sites of L. enigmaticus and the lydiae form of major L. reichei are at an altitude of 2200–2400 m (Neita-Moreno & Ratcliffe, 2019). Interestingly, positive allometry of EH/EW, which implies that major males have more convex elytra, is a phenomenon of individuals who dwell at higher altitudes (Mani, 1968).
Allometry of L. reichei
In this study, CHL/BCHL and THL/PW were highly correlated with respect to BL. Furthermore, the two ratios were significantly different between the major and minor groups, which implies that both the horns grew very rapidly with an increasing body size. Dramatic polymorphism of L. reichei originates from these traits; basal tooth of the cephalic horn and thoracic horn became prominent sight along with the body size. As Moczek suggested, body size is critical for horn development, and the body sizes depend on the nutritional status during the larva stage (Moczek, 2002). The tendency that the majority of collected specimens possess minor traits may infer that environmental constraints exist in the habitants of larvae of L. reichei.
The relation between body length and horn length is not linear, rather it is discontinuous—this threshold that separates morphologies was observed. As seen in the principal components analysis results in this study, individuals could be clearly clustered into three groups. BL of the minor groups was 20.99–25.16 mm, that for the medium group was 25.04–26.1 mm, and that for the major group was 29.18–31.35 mm. Discontinuous points of the thoracic horn growth occurred at 25.04–25.16 mm and 26.1–29.18 mm. At the former point, the apex of the thoracic horn started to fold, and, at the latter point, the thoracic horn exhibited a major characteristic. A similar tendency was observed with respect to the basal tooth of the cephalic horns.
Interestingly, among the analyzed traits, only ED displayed a negative correlation with the other traits. Despite the interocular distance not being any different between the major and minor males (p = 0.57, data not shown), EW was strikingly smaller in minor males (p = 0.00006, data not shown). In contrast, Onthopagus acuminatus and O. taurus were individuals with larger horns and smaller eyes, and vice versa (Emlen & Nijhout, 1998). This phenomenon was explained by the tradeoff hypothesis, that is, individuals with large horns and large eyes are difficult to produce due to the limitations in resources (Emlen, 2000). Kawano reported that wing length and width were negatively allometric to body size in some Dynastinae, such as Chalcosoma chiron, C. atlas, Dynastes neptunus, and D. hercules (Kawano, 1995). Although, in L. reichei, there is a tendency for EL to be shorter in major males, it was not statistically significant. These results indicate the presence of evolutional pressure that is yet to be described.
Figures and Tables
Fig. 3
Measuring strategy of each body part. BCHL, basal cephalic horn length; CHL, cephalic horn length; TH, tooth height; THL, thoracic horn length; EH, elytra height; ED, eye distances; PW, pronotum width; EL, elytra length; BL, body length.
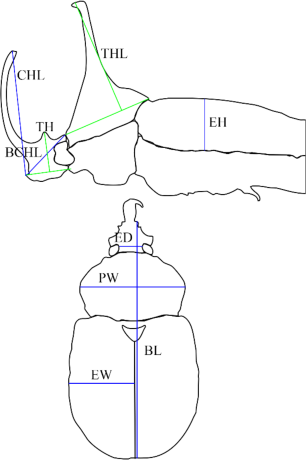
Fig. 4
Allometry of horns of L. reichei and L. hirtipes. A. L. reichei major male, lydiae form; B. L. reichei major male, holotype, reichei form; C. L. reichei in major group, reichei form; D. L. reichei in major group, reichei form; E. L. reichei minor male; F. L. hirtipes.

Fig. 5
Box plots showing ratios of L. reichei major male and minor male. BCHL, basal cephalic horn length; CHL, cephalic horn length; TH, tooth height; THL, thoracic horn length; EH, elytra height; ED, eye distances; PW, pronotum width; EL, elytra length; BL, body length.
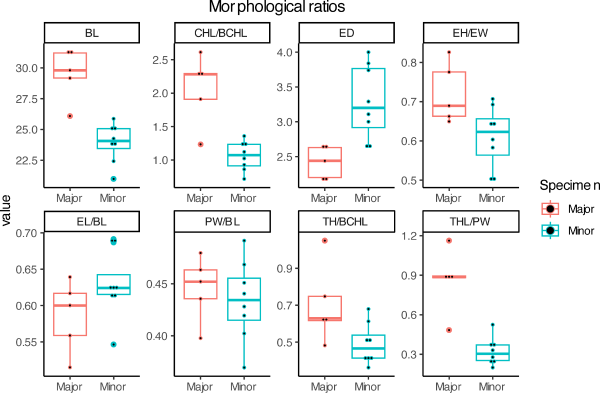
Table 1
Information about the studied specimens
| Studied specimen | Collected location | Collected date | Collector | Institute or collection |
|---|---|---|---|---|
| lydiae_holotype | Guacamayo, Colombia | 12, 2011 | Unknown | CPFA |
| EC11814 | Colombia | Unknown | Unknown | MNHN |
| reichei_holotype | Socorra, Colombia | Unknown | Unknown | NHM |
| WCPC41 | La india, Colombia | 04, 2022 | Unknown | WCPC |
| WCPC42 | La india, Colombia | 04, 2023 | Unknown | WCPC |
| WCPC43 | La india, Colombia | 04, 2024 | Unknown | WCPC |
| WCPC44 | La india, Colombia | 04, 2025 | Unknown | WCPC |
| WCPC45 | La india, Colombia | 04, 2026 | Unknown | WCPC |
| WCPC46 | La india, Colombia | 04, 2027 | Unknown | WCPC |
| WCPC48 | La india, Colombia | 04, 2028 | Unknown | WCPC |
| WCPC49 | La india, Colombia | 04, 2029 | Unknown | WCPC |
| WCPC73 | Santander, Colombia | 04, 2019 | Unknown | WCPC |
| WCPC74 | Otanche, Colombia | 05, 2021 | Unknown | WCPC |
| WCPC75 | Valle del Cacuca, Colombia | 25.03.2017 | V. Sinyaev | WCPC |
| WCPC77 | Valle del Cacuca, Colombia | 10, 2023 | Pablo S. Wagner | WCPC |
| WCPC78 | Valle del Cacuca, Colombia | 10, 2024 | Pablo S. Wagner | WCPC |
| WCPC79 | Valle del Cacuca, Colombia | 10, 2025 | Pablo S. Wagner | WCPC |
| WCPC80 | Santander, Colombia | Unknown | Unknown | WCPC |
| WCPC81 | Santander, Colombia | 03.03.2017 | Unknown | WCPC |