Introduction
Zoos are a means to carry out conservation of wildlife and provide education and awareness amongst the public. They, hence, need to ensure the physical and mental well-being of captive animal populations that support the existing population in the wild. Provision of an optimal animal welfare at a captive facility fosters comfortability, safety, ability to express innate and natural behaviours, and prevents distress. However, any external environmental stressor may lead to an alteration in the way an animal copes with its surroundings (Morgan & Tromborg, 2007). Reduced life expectancy, diminished growth, impaired reproduction, diseases, behaviour anomalies, and body damage may result when one is unable to endure the sub-optimal welfare (Broom, 1991).
Captive conditions are vastly different from the wild environments in terms of spatial confinement, restrictions, simplicity, control, and predictability (Morgan & Tromborg, 2007). Zoo-housed animals are thus compelled to mechanisms to cope up with such monotonous surroundings (Mason, 1991). Stress and frustration in captivity is suggested by the variety of stereotypic behaviours induced amongst animals. They often perform repetitive, abnormal behaviours such as pacing, coprophagy, overgrooming, and head-weaving (Lyons et al., 1997). Stereotypies may arise from a primary behaviour pattern that caged species have eventually become motivated to perform (Holzapfel, 1939; Mason et al., 2007). Such behaviours are a strategy to pass time and substitute free-ranging behaviour as they have no apparent function or goal (Carlstead, 1998; Hediger, 2013; Odberg, 1978). They generally indicate the suboptimal level of an animal’s psychological welfare (Boorer, 1972; Mason, 1991).
Humans tend to leave an impact on their surrounding environment. Visitors at the zoological parks form relationships with captive animals and are recorded to often induce alterations in their behaviour repertoire (Cole & Fraser, 2018; Davey, 2007; Hosey, 2000). The “visitor effect” could be positive, neutral, or negative (Hosey, 2008; Hosey & Melfi, 2015). When human interactions benefit the caged animal and increase the animal’s species-specific behaviour, they foster positive and healthy relationships with animals (Baker, 2004; Claxton, 2011). This results in a significant reduction in the time spent performing stereotypies and inducing natural or wild-type behaviours. There may also be certain conditions when animals become habituated to visitors due to consistent exposure and thereby exhibit no behavioural changes. On the other hand, the visitor’s unfitting behaviours can result in an adverse effect of visitation. Activities such as shouting, teasing, throwing stones, hitting, and moving in unpredictable ways can impel fear and stress response in captive animals (Cole & Fraser, 2018; Mallapur, 2004; Venugopal & Sha, 1993). The mere presence of human visitors yields a significant impact on the behaviour of various mammalian species in zoos (Hosey, 2000). Different visitor attributes, such as presence, density, activity, noise, and proximity, can influence captive individual’s behaviour and physiology (Brouček, 2014; Davey, 2005; 2007). Prevalence of stereotypy may intensify on the days of a large and noisy human audience (Dybowska et al., 2008; Mallapur & Chellam, 2002; Vidal et al., 2016).
The activity pattern of an animal is an expression in response to the resources available in surroundings, and hence in zoos, animals display distinct behaviours as compared to those in the wild (Young, 2003). Behaviour studies, in relation to the knowledge of species-specific behaviours in the wild, help to assess the welfare of zoo-housed animals (Keeling & Jensen, 2002). The behaviour of animals reflects its first attempt to cope with sub-optimal environmental conditions and hence acts as an effective useful welfare indicator (Bashaw et al., 2003; Dawkins, 1998). Studying the extent to which visitation and other captive factors influence captive species’ behaviour is a non-invasive measure. It is pivotal for suggesting better management practices for upkeep and welfare.
National Zoological Park (NZP) is one of the prominent Indian zoos. It entertains a large number of visitors each year. From 2014-19, an average of 2.36 million people paid a visit to the zoo each year (National Zoological Park, 2019). Big cats like tigers (Panthera tigris) and leopards (Panthera pardus) are major attractions for the most public. Visitors are more attentive and spend a long time viewing the animal when they are active and display species-specific behaviours (Altman, 1998; Bitgood et al., 1988; Fernandez et al., 2009; Margulis et al., 2003).
Being a notable zoo, understanding of the welfare of captive species at the NZP is of utmost importance. The extent to which captivity and visitation have an impact on the behaviour of some magnificent felid species has not been studied in detail yet. Hence, the study was designed to understand the activity budget and prevalence of stereotypic behaviours amongst captive tigers (Panthera tigris) and leopards (Panthera pardus) housed at NZP, New Delhi. Further, it aimed to identify the influence of biological, captive, and visitation factors on stereotypy. The effect of enclosure size and design, enrichment, crowd size, and ambient noise was studied on the stereotypic behaviours performed by the captive subjects. Additionally, animal history factors like age, sex, colour coat, and breeding history were also considered as factors impacting stereotypy.
Materials and Methods
Ethics approval
The study involves no animal capture and handling and thus does not require any animal ethics committee permission. However, the necessary permission was obtained from concerned authorities to conduct the study. The subjects were monitored from a distant place without disturbing their natural behaviour.
Study area and subjects
NZP, New Delhi, received the status of being the model zoo for the entire country (Agnihotri, 2012). The zoo spreads over 188.62 acres of land with 72 enclosures and houses 99 species with around 1,200 inmates. It boasts a distinguished history of successful breeding of various animals including tiger (Panthera tigris), brow-antlered deer (Rucervus eldii), Indian rhinoceros (Rhinoceros unicornis), and Asiatic lion (Panthera leo) (Agnihotri, 2012).
We studied a total of eight individually-housed subjects, including four individuals each of tiger (Panthera tigris) and leopard (Panthera pardus) (Table 1). Three of the tigers were white and one a normal variant. All three white tigers were housed in one enclosure with an arena area of 1,445 m2. The studied normal tiger was housed in an enclosure of about 858 m2 arena area. The leopards were housed in two adjacent outdoor enclosures with an arena area of about 158 m2 and 136 m2. The animals were moved to indoor enclosures during off-exhibit hours, i.e., in evening and on off days. No visitors are allowed to view animals in the indoor enclosures. The animals were fed with buffalo meat once a day in their retiring night cells, except Fridays. Individuals on exhibit were fed during the evening hours, while the rest were served in the afternoon. The on-exhibit areas for tigers were furnished with logs, trees, vegetation, pool, and water supply. While for leopards, the enclosures were provided with logs and barks to climb, vegetation, and water. None of the eight subjects have been provided with meticulous enrichment programs in the past, besides the basic elements set in the enclosures. The off-exhibit or indoor enclosures were simple in nature, with no enrichment. A pair of male and female leopards was let out into adjacent on-exhibit enclosures together. Subjects studied were let out in the on-exhibit enclosure from 09:30 to 16:30 hours. Since no animals were released in the outdoor enclosure on Friday, the subjects were studied for six days a week.
Activity budgeting
The study was conducted for 208 hours, during the summer months of 4 May and 17 June in 2019. A pilot study of three days (23 April 2019-25 April 2019) helped to enlist all the behaviours performed by the two species. The average temperature during the study period was noted to be approximately 37°C. Each individual was observed for 6 hours per day, for 4 consecutive days. Observations began from 9:00, when the zoo was opened and the cats were let into the on-exhibit enclosures. They lasted till 16:30, when the zoo closed and the animals were transferred to indoor enclosures. The period was divided into four observation session blocks: 9:00-10:30, 11:00-12:30, 13:00-14:30, and 15:00-16:30. Focal animal behavioural sampling at 1-minute intervals was used to construct an ethogram of the big cats (Altmann, 1974). The interval of 1-minute was set as behaviours of felids altered swiftly, as observed during the pilot study (Biolatti et al., 2016). In case the behaviours were continued for a longer duration, the same activity was marked in the checklist. The observations were made from the visitor area, which was at an approximate distance of three to five metres from the enclosures. The activities performed were classified into three categories - a) active, b) inactive, c) stereotypic, for comparison and analysis (Table 2). Active behaviours include activities such as climbing, cooling, drinking, eating, excreting, grooming, licking, olfaction, playing, rubbing, rolling over, running, scratching, scent marking, vocalisation, and walking (Appendix). Activities such as lying on back, resting awake, sitting, sleeping, and standing are classified under inactive behaviours. Stereotypic behaviours were performed in various forms, including pacing, skip-pacing, and tail or toe sucking. The behaviour was considered pacing when the animal covered three or more traverses of a definite path (Forthman & Bakeman, 1992). The enclosures were divided into different zones – middle, edges, and visitor areas. Zone utilized for each state of behaviour by animals was also recorded.
Biological and captive factors
The history of all the studied individuals was obtained from Zoo Aquarium Animal Management Software (ZIMS; Species360, Bloomington, MN, USA) which records a studbook database. For tigers, biological aspects such as age, sex, breeding history, and coat colour were recorded (Table 1). While for leopards, age and sex were taken in consideration. Age was classified as young/adult; sex as male/female; breeding history as bred/unbred; and coat colour as mutant/normal. Classification of the species on the basis of done as per the age class referred the in the Field Guide for Aging Tigers (Jhala & Sandhu, 2017). Enclosure size and design was considered as the captive factor for both species. Enclosures were categorised as small and simple and large and complex, based on the guidelines of Central Zoo Authority of India (Bonal et al., 2014). Association of these variables was evaluated with stereotypic levels of all tigers and leopards.
Visitation
Visitation and behaviour data were collected simultaneously. At the interval of 1 minute, the number of visitors were counted and recorded. This was in accordance to the pilot study, during which the average time spent by a visitor at an enclosure was found to be approximately 1 minute. Visitor density (crowd size) was calculated by counting the number of visitors standing at the visitor area around the enclosures.
Statistical analysis
The frequency of each behavioural activity was calculated by converting it to the percentage of time devoted to the respective behaviour. Time spent in performing inactive, active, and stereotypic behaviours was also calculated and expressed as percentage. Frequency of behaviours in each of three categories were also quantified according to the 4 sampling intervals spanning throughout the day. Stereotypic and non-stereotypic (inactive and active) behaviours were represented as strings of 0’s and 1’s, where 1 denoted presence of stereotypy and 0 denoted absence of stereotypy. Activity budget and stereotypy prevalence were recorded and analysed in Microsoft Excel 2019 (Microsoft, Redmond, WA, USA). The average visitor density per minute and average ambient noise per minute at the cage was calculated.
For further statistical analysis, IBM SPSS for Windows (version 23; IBM Co., Armonk, NY, USA) was used. The behavioural data presented a non-normal distribution hence, the relationship with factors was investigated using Generalised Linear Model (GLM) with Binomial distribution and log link function (Zuur et al., 2009). For the model, behaviour was used as dependent variable, visitor density as covariate, and others as factors. In all the tests, P-value (α) was defined at the value of 0.05 to establish statistical significance.
Results
Activity budgeting and prevalence of stereotypy
The activity budget of tigers (n=4) revealed that they devoted a significant amount of time displaying inactivity (48.5±15%), of which, sitting was the most common (23.8±7%) (Table 2). Animals utilized the middle area and edges of enclosures for inactive behaviours. Tigers devoted 31.3±8% of their time to active behaviours (Fig. 1). Cooling was the most performed behaviour (8.5±5%), followed by walking (4.7±2%). The middle, enriched zones of the enclosures were generally utilized for active behaviours. Tigers performed stereotypy for 20±13% of their time. Pacing (19.9±13%) and tail or toe sucking (0.1±0.1%), were the two forms of stereotypic behaviours. The pacing was predominantly performed toward the edges of the enclosure. Active behaviours (42.8±17%) were found to be much higher during the morning hours (9:00-10:30) in comparison to other time intervals (Fig. 2). Inactive behaviours, on the contrary, were the highest (60±10.6%) during afternoon hours (13:00-14:30). Tigers performed the highest frequency of stereotypic behaviours (27.4±16%) in morning hours (9:00-10:30), followed by evening hours, 15:00-16:30, (24.9±19%).
Like tigers, leopard subjects also devoted a considerable amount of time to inactive behaviours (58±13%, Fig. 1). Sitting was the most common inactive behaviour (45.2±6%, Table 2). The middle and rear areas of the enclosure were generally utilized for inactivity. They display active behaviours only for about 18.1±2% of their time in on-exhibit enclosure. The highest amount of time was devoted to walking (5.9±1%) of active behaviours (Table 2). Enriched zones of the enclosures were found to be utilized during the active periods. Leopards spent about 25.5±13% of their time in displaying stereotypy. Unlike tigers, leopards display a more varied form of stereotypy, including pacing (25±12%), skip-pacing (0.8%), and tail or toe-sucking (0.3%) (Table 2). They paced toward the edges and visitor zone of the enclosure. Leopards exhibited high active behaviours (25.6±3%) during the morning hours (9:00-10:30) as compared to other time intervals (Fig. 3). Inactive behaviours were much higher (63.8±17%) during the afternoon hours (13:00-14:30). Stereotypic behaviours were most common during the morning hours, from 9:00-10:30 (33.5±19%), followed by the evening interval, 15:00-16:30 (29.1±17%).
Biological and captive factors
The effect of biological factors (age, sex, breeding history, and coat color) and captive factor (enclosure design and size) on prevalence of stereotypy was analysed. GLM with binomial distribution and log link function was employed to test the influence. In both species, adults (tiger=27.85±5.45%; leopard=30.66±7.81%) performed much more stereotypy in comparison to young (tiger=6.7%; leopard=12.56%) (Table 3). The relationship between age and stereotypy was found significant for tigers (Wald χ2=30.14; df=1; P<0.05) and leopards (Wald χ2=25.58; df=1; P<0.05). A significant effect of sex was revealed for tigers (Wald χ2=12.97; df=1; P<0.05) and leopards (Wald χ2=80.92; df=1; P<0.05). Males exhibited a high level of stereotypy (tigers=27.85±5.45%, leopards=37.66±0.81%) as compared to females (tigers=16.9±10.2%, leopards=17.7±5%). Analysis of stereotypy with respect to breeding history revealed a significant difference (Wald χ2=39.51; df=1; P<0.05) in tigers as bred subjects (n=3) displayed more stereotypic behaviours (27.8±5%) than unbred animals (6.7%). Coat color was also found to influence tigers’ stereotypy (Wald χ2=30.86; df=1; P<0.05) as normal coated tigers (27.1%) displayed more stereotypy than white tigers (20.8±13.3%). Tigers housed in different enclosures revealed a significant variation in stereotypic levels (Wald χ2=51.31; df=1; P<0.05). Those housed in small and simple enclosures displayed more stereotypy (27.1%) in comparison to those in large and complex enclosures (20±13.3%). Leopards housed in an enclosure with smaller area (n=2) performed slightly higher levels of stereotypy (29.85±7%) as compared to those in a larger enclosure (25.51±12.95%). However, the relationship was not statistically significant (Wald χ2=0.23; df=1; P>0.05) (Table 3).
Visitation effect
The average density of the visitors was found higher around tiger enclosures (31 humans/minute) compared to leopards (13 humans/minute). Visitor density was found to the highest during evening hours for tigers (44±8 humans/minute; 67±1 dB/minute) and leopards (17±6 humans/minute; 64±1 dB/minute).
GLM with binomial distribution and log link function was employed to test the effect of visitor density on prevalence of stereotypy amongst both species. For visitor density, it revealed a significant influence on stereotypic behaviours in tigers (B 0.06±0.02; Wald χ2 24.32; df=1, P<0.05) and leopards (B 0.02±0.003; Wald χ2 27.90; df=1; P<0.05). The results reveal that visitor density is a sound predictor and stereotypic behaviours increase with increase in visitor density (Wald χ2=24.32 for tigers; Wald χ2=27.90 for leopards; df=1; P<0.05) (Table 4).
Discussion
In response to any change in the environment, alteration of behaviour repertoire reflects the first line of defence of an animal. High proportions of inactive behaviours found in the study align with various activity budgeting studies (Biolatti et al., 2016; Mallapur & Chellam, 2002; Pitsko, 2003; Sajjad et al., 2011; Yu et al., 2009). Lack of enrichment elements and hiding refuge in captive conditions may cause excessive inactivity (Mallapur & Chellam, 2002). In this study, all subjects performed the stereotypical behaviours for 7% to 38% of the time in varying forms like pacing, skip-pacing, and tail or toe sucking (Fig. 1 and Table 2). Stereotypic pacing is usually accompanied by consistent behaviour of marking territory (Boorer, 1972). The high proportion of stereotypy amongst captive felids has been demonstrated in multiple studies (Bashaw et al., 2003; Biolatti et al., 2016; Clubb & Mason, 2007; De Rouck et al., 2005; Mallapur et al., 2002; Mohapatra et al., 2014; Sajjad et al., 2011). It has been suggested that the stereotypical level beyond 10% of the total activity is generally unacceptable for any captive animals (Broom, 1983). According to some studies, an animal’s welfare status is considered unacceptable if more than 5% of the studied population performs stereotypic behaviours (Mason, 1991; Wielebnowski, 2003). In predatory animals, stereotypies are generally locomotory in nature, which may result from their motivation to forage, range, seek mate, patrol territory, explore, and escape aversive situations (Clubb & Vickery, 2006). The significant pacing levels along enclosure edges were also reported by other studies involving various felid species (Lyons et al., 1997; Mallapur et al., 2002; Sajjad et al., 2011). Leopards exhibited broader range of stereotypic behaviours in comparison to tigers, which could be due to their small-sized enclosures. As the leopard enclosures offered them limited space to perform pacing, the stereotypy was elicited in other forms like skip-pacing and tail or toe sucking.
Animals exhibit a high degree of stereotypic behaviours during the morning hours (9:00-10:30), which could be due to the natural instinct to patrol and forage. Intensified pacing and restlessness coincided with feeding and when the food truck was audible or visible to the animals. Bouts of stereotypy also overlapped with the presence of animal keepers around the housing exhibits. Numerous studies on big cats made identical observations (Mohapatra et al., 2010; Mohapatra et al., 2014; Palita, 1997). The high stereotypies displayed by the two species could be induced by the predictable feeding regime and simplified food provisioning technique. Modification of food, such as hiding it or an unpredictable schedule, can enhance the targeted animal welfare (Shepherdson et al., 1993; Watters et al., 2011).
This study suggested the increased display of stereotypic behaviour in males compared to female conspecifics. Few other studies have reported similar influence of sex on stereotypy in case of captive felids (Dybowska et al., 2008; Vaz et al., 2017). Due to the male big cat’s larger territory size in the wild, the male individuals may experience more spatial stress in enclosed spaces. Normal coated tigers displayed more stereotypy than white or mutant-coated tiger. Tigers bred in captivity exhibited more stereotypy. Similar findings were also reported in another study (Vaz et al., 2017). Adult tigers and leopards showed more stereotypic behaviours than young counterparts, as recorded in other studies (Breton & Barrot, 2014; Vaz et al., 2017). This is supported by the motion that stereotypies develop when felids become old enough to disperse from their natal home range and further intensify with age (Mohapatra et al., 2014; Smith, 1993). As animal ages and body enlarges, it experiences spatial constraints in captive conditions, causing behavioural repertoire alterations.
Visitor presence, the noise produced, visitor proximity and behaviour, are known to influence captive species’ behaviour repertoire (Hosey & Druck, 1987). Human activities such as shouting, teasing, banging barriers, and throwing stones at the animals may cause psychological and physical harm to the victim animal (Venugopal & Sha, 1993). Visitor effect could induce stress in zoo animals, which may ultimately contribute to the appearance of pathologies and failure of captive breeding programs (Carder & Semple, 2008; Chamove et al., 1988; Hosey & Druck, 1987). The study revealed the negative impact of visitor crowd size on the behaviour repertoire of tigers and leopards. As the audience size increased during the evening hours (15:00-16:30), the degree of stereotypy performed by animals also increased. A large visitor crowd has shown to influence stereotypy of captive felid species in various studies (Quadros et al., 2014; Sellinger & Ha, 2005; Vidal et al., 2016). Leopards housed in enclosures with larger viewing area performed high levels of stereotypic behaviours, thus supporting the effect of visitation on big cats in captivity. Bouts of stereotypy due to visitors’ presence suggest the animal’s motivation to express flight behaviour, but unable to perform the desired behaviour (Dembiec et al., 2004).
One of the primary purposes of zoological parks is to impart knowledge and the idea of conservation amongst the public. Zoos need to attract visitors and communicate a strong message of conservation to achieve this. Visitor attraction hypothesis suggests that active animals engage visitors more efficiently, while inactive and stereotypic behaviours performed by animals are perceived as boredom and stress by the visitors (Hosey, 2000). Therefore, it is crucial to alleviate the sub-optimal captive conditions to promote active behaviours amongst the captive big cats. The provision of enrichment techniques is a mean to reduce levels of stereotypy and inactivity in captive felines (Mallapur et al., 2002; Powell, 1995; Skibiel et al., 2007). Provision of cardboard box and toys, hiding refuge, elevated platforms, and olfactory enrichment are few recommended enrichment techniques to ensure optimal welfare (Bashaw et al., 2003; Damasceno et al., 2017; Jenny & Schmid, 2002; Markowitz & LaForse, 1987; McPhee, 2002; Mellen & Shepherdson, 1997; Mohapatra et al., 2010). Such techniques aid to encourage feeding, exploration, and interaction by eliciting species-specific behaviours. Moreover, the installation of appropriate visual barriers between caged animals and visitors is also an efficient measure to reduce the prevalence of stereotypic behaviours (Blaney & Wells, 2004).
This study suggests that the stereotypic behaviours were prevalent amongst tigers and leopards at NZP, New Delhi. The levels of stereotypy differed for the biological and captive factors of the big cats. Male, adult, and previously bred individuals exhibited the lengthened pacing periods compared to female and young individuals. Stereotypic behaviours performed by captive tigers and leopards were significantly impacted due to visitation. Large audience size led to an increase in the proportion of time spent in performing stereotypy. However, a small sample size of only four individuals for each species limits the study despite statistically significant results. Availability of a higher number of sampled individuals would produce reliable and definite results. Despite the limitations, we recommend the installation of visual barriers to minimize the viewing area. Although enclosures at NZP follow the norms laid by the Central Zoo Authority, providing enrichment may possibly reduce the stereotypical behaviours and enhance the captive species’ welfare. Enrichment elements such as hidden spots and refuges, which mimic the wild, may promote the animals to exhibit more exploratory behaviours.
Acknowledgments
The authorities of the Amity Institute of Forestry and Wildlife, Amity University, and National Zoological Park, New Delhi, are acknowledged for their administrative support and permission to carry out the research work. We wish to thank the zoo staff of National Zoological Park for their assistance during the field study. The study is partially supported by the Bio-bridge Initiative (2019-20) research grant of the Secretariat of the Convention on Biological Diversity and the Ministry of Environment of the Republic of Korea.
Author Contributions
AG and PP conceived and designed experiments. UH and HL improvised the experiment design and assisted in data analysis. AG, SV, and MS acquired the data. AG analysed data and wrote the manuscript. All authors read and approved the final version of the manuscript.
References
National Zoological Park (2019, Retrieved Mar 4, 2022) Annual Report 2018-19 from http://cza.nic.in/uploads/documents/reports/english/AR_delhizoo_1819.pdf
Figures and Tables
Fig. 1
Proportion of time spent by tigers and leopards in performing behaviours – inactive, active, and stereotypic.
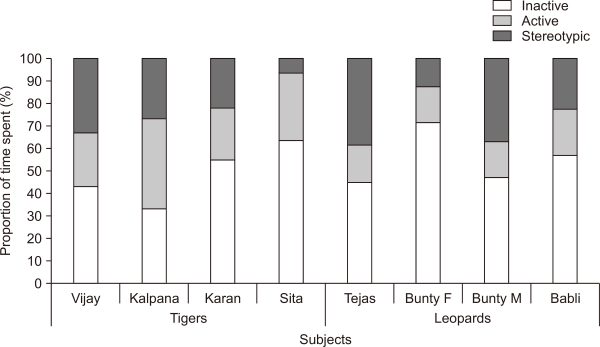
Fig. 2
Proportion of time spent in performing inactive, active, and stereotypic behaviours during four time intervals of the day by tigers.
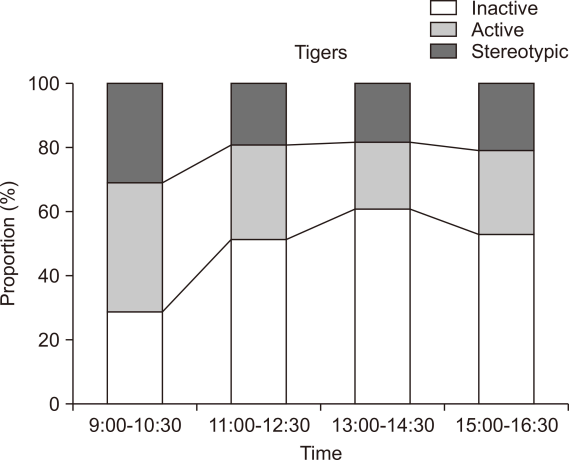
Fig. 3
Proportion of time spent in performing inactive, active, and stereotypic behaviours during four time intervals of the day by leopards.
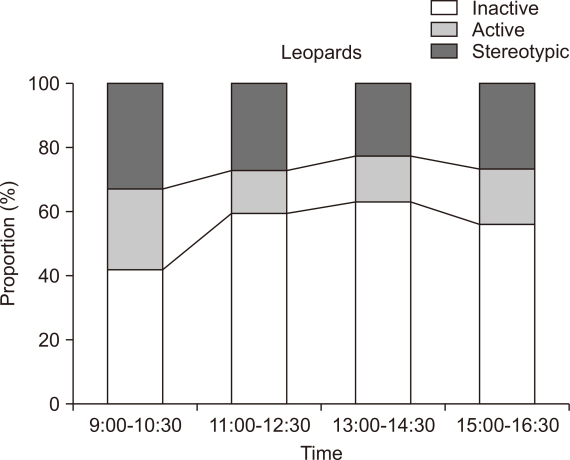
Table 1
Subjects studied and their history (ZIMS) at National Zoological Park
| Individual | Coat colour | Sex | Age (years)* | Origin | Rearing history |
|---|---|---|---|---|---|
| Tiger (Panthera tigris) | |||||
| T_M1 | Mutation | Male | 12 | Captive (Delhi Zoo) | Parent |
| T_F1 | Mutation | Female | 12.4 | Captive (Delhi Zoo) | Parent |
| T_M2 | Normal | Male | 6 | Captive (Mysore Zoo) | Parent |
| T_F2 | Mutation | Female | 4.3 | Captive (Delhi Zoo) | Unbred |
| Leopard (Panthera pardus) | |||||
| L_M1 | Normal | Male | 6.8 | Wild (Uttarakhand) | - |
| L_F1 | Normal | Female | 2.25 | Wild (Jammu) | - |
| L_M2 | Normal | Male | 7.9 | Wild (Chhattisgarh) | - |
| L_F2 | Normal | Female | 8.8 | Wild (Chhattisgarh) | - |
Table 2
Percent time spent on various activities by individual tigers & leopards at National Zoological Park
| Activity | Tigers | Leopards | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| T_M1 | T_F1 | T_M2 | T_F2 | L_M1 | L_F1 | L_M2 | L_F2 | ||
| LBi | 0 | 0 | 0 | 0 | 0.06 | 0 | 0.06 | 0.65 | |
| RAi | 0.45 | 0.65 | 2.6 | 2.47 | 0.26 | 1.04 | 0.26 | 3.06 | |
| SIi | 16.4 | 24.6 | 21.8 | 31.18 | 39.45 | 50.97 | 39.97 | 47.58 | |
| SLi | 21.1 | 4.16 | 26.04 | 24.15 | 2.41 | 16.86 | 5.53 | 2.08 | |
| STi | 5.4 | 3.9 | 4.3 | 5.8 | 2.93 | 2.08 | 1.3 | 3.58 | |
| CLa | 0 | 0 | 0 | 0.45 | 0.71 | 0.12 | 0 | 0.91 | |
| COa | 8.3 | 13.28 | 10.8 | 3.78 | 0 | 0 | 0 | 0 | |
| DRa | 2.15 | 3.06 | 0.19 | 1.45 | 0.85 | 0.45 | 0.58 | 0.97 | |
| EAa | 1.17 | 8.6 | 0.05 | 6.18 | 2.08 | 4.1 | 1.5 | 2.21 | |
| EXa | 0.26 | 0.26 | 0.13 | 0.19 | 0.19 | 0.19 | 0.19 | 0 | |
| GRa | 0.32 | 0.32 | 3.7 | 2.14 | 1.3 | 1.23 | 0 | 0.71 | |
| LIa | 0.59 | 0.59 | 0.13 | 1.82 | 0.19 | 0.52 | 1.17 | 0.32 | |
| OLa | 0 | 0 | 0 | 0.05 | 0.06 | 0.13 | 0.85 | 0.13 | |
| PLa | 0.13 | 0.13 | 0.26 | 0.05 | 0.26 | 0 | 0.26 | 1.1 | |
| RBa | 1.17 | 1.17 | 0 | 0.78 | 0,.52 | 0.71 | 0.52 | 1.17 | |
| ROa | 0 | 0 | 0 | 0 | 0.45 | 0.52 | 0.45 | 1.56 | |
| RUa | 0 | 0 | 0 | 0 | 0.58 | 2.15 | 0 | 2.08 | |
| SCa | 0.06 | 0.06 | 0.05 | 0 | 0 | 0.52 | 0.26 | 0.06 | |
| SMa | 4.49 | 4.49 | 1 | 3.32 | 2.41 | 0 | 3.15 | 0.13 | |
| VOa | 2.35 | 2.35 | 1.1 | 2.55 | 0.65 | 0.52 | 0.13 | 1.95 | |
| WLa | 2.4 | 2.4 | 5.5 | 7 | 6.12 | 5.27 | 6.57 | 6.31 | |
| PAst | 33.2 | 33.2 | 22.25 | 6.64 | 36.26 | 12.5 | 36.85 | 22.72 | |
| SPst | 0 | 0 | 0 | 0 | 1.63 | 0 | 0 | 0 | |
| TSst | 0.06 | 0.06 | 0.1 | 0.05 | 0.58 | 0.06 | 0 | 0.13 | |
LB, lying on back; RA, resting awake; SI, sitting; SL, sleeping; ST, standing; CL, climbing; CO, cooling; DR, drinking; EA, eating; EX, excreting; GR, grooming; LI, licking; OL, olfactory behaviour; PL, playing; RB, rubbing; RO, rolling over; RU, running; SC, scratching; SM, scent marking; VO, vocalization; WL, walking; PA, pacing; SP, skip pacing; TS, tail/toe sucking; i, inactive behaviour; a, active behaviour; st, stereotypy.
Table 3
Generalised Linear Models to identify association of stereotypic behaviours displayed by captive tigers and leopards with various variables
| Species | Independent factors | Time spent on behaviour(mean±SE)% (n=6,144) | Statistical test result | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Non-stereotype | Stereotype | Wald χ2 | P-value | ||||
| Tiger | Age | Adult | 72.15±5.45 | 27.85±5.45 | 30.14 | 0.001 | |
| Young | 93.3 | 6.7 | |||||
| Sex | Male | 72.5±5.45 | 27.85±5.45 | 12.97 | 0.001 | ||
| Female | 83.1±10.2 | 16.9±10.2 | |||||
| Breeding history | Bred | 72.15±5.45 | 27.85±5.45 | 39.51 | 0.001 | ||
| Unbred | 93.3 | 6.7 | |||||
| Coat colour | White | 80±13.3 | 20±13.3 | 30.86 | 0.001 | ||
| Normal | 72.9 | 27.1 | |||||
| Enclosure | Large | 80±13.3 | 20±13.3 | 51.31 | 0.001 | ||
| Small | 72.9 | 27.1 | |||||
| Leopard | Age | Adult | 69.34±7.81 | 30.66±7.81 | 25.58 | 0.001 | |
| Young | 87.44 | 12.56 | |||||
| Sex | Male | 62.34±0.81 | 37.66±0.81 | 80.92 | 0.001 | ||
| Female | 82.29±5.14 | 17.7±5 | |||||
| Enclosure | Large | 74.48±12.95 | 25.51±12.95 | 0.23 | 0.632 | ||
| Small | 70.15±7 | 29.85±7 | |||||
Table 4
Generalised Linear Model to understand influence of visitation on stereotypic behaviours of tigers and leopards
| Species | Independent factor | Average per minute(n=6,144) | Statistical test result | |||
|---|---|---|---|---|---|---|
|
|
||||||
| B | Wald χ2 | df | P-value | |||
| Tiger | Density | 31 humans/minute | 0.06±0.02 | 24.32 | 1 | 0.001 |
| Leopard | Density | 13 humans/minute | 0.02±0.003 | 27.90 | 1 | 0.001 |