 E-ISSN : 2671-6771
E-ISSN : 2671-6771
Mucopolysaccharidosis Type III: review and recent therapies under investigation

Abstract
Mucopolysaccharidosis type III (MPS III or Sanfilippo syndrome) is a multisystem lysosomal storage disease that is inherited in an autosomal recessive manner. It consists of four subtypes (MPS IIIA, B, C, and D), each characterized by the deficiency of different enzymes that catalyze the metabolism of the glycosaminoglycan heparan sulfate at the lysosomal level. The typical clinical manifestation of MPS III includes progressive central nervous system (CNS) degeneration with accompanying systemic manifestations. Disease onset is typically before the age of ten years and death usually occurs in the second or third decade due to neurological regression or respiratory tract infections. However, there is currently no treatment for CNS symptoms in patients with MPS III. Invasive and non-invasive techniques that allow drugs to pass through the blood brain barrier and reach the CNS are being tested and have proven effective. In addition, the application of genistein treatment as a substrate reduction therapy is in progress.
- keywords
- MPS III, Sanfilippo syndrome, blood brain barrier, Treatment, Genistein
INTRODUCTION
Mucopolysaccharidosis type III (MPS III or Sanfilippo syndrome) is an autosomal recessive, multisystem lysosomal storage disease that is characterized by progressive central nervous system (CNS) degeneration manifested in the form of severe intellectual disability, developmental regression, autism spectrum disorder, behavioral problems, and sleep disturbances, with death usually in the mid-teens to early twenties [1]. Accumulation of glycosaminoglycans (GAGs) in the CNS can seriously affect neurons, leading to death through apoptosis or necrosis during the advanced stages of the disease [2]. The incidence of MPS III is estimated to be 1 in 70,000 live births, while the overall point prevalence varies with geographical area, but is estimated to be 1 to 9 in 1,000,000 people [3]. MPS III consists of 4 subtypes (MPS IIIA to D), each characterized by the deficiency of different enzymes that catalyze the metabolism of the GAG heparan sulfate (HS) at the lysosomal level (Table 1).
Table 1.
The genes and enzymes associated with each MPS III subtypes
| Subtype | Gene | Deficient enzyme | Locus |
|---|---|---|---|
| MPS IIIA | SGSH | N-sulphoglucosamine sulphohydrolase | 17q25.3 |
| MPS IIIB | NAGLU | Alpha-N-acetylglucosaminidase | 17q21.2 |
| MPS IIIC | HGSNAT | Heparan-alpha-glucosaminide N-acetyltransferase | 8q11.21 |
| MPS IIID | GNS | N-acetylglucosamine-6-sulfatase | 12q14.3 |
The prevalence of MPS varies with geographical area and certain subtypes appear to be predominant in specific regions of the world. Overall, MPS III types A and B are more commonly diagnosed than types C and D. The estimated incidences for subtypes MPS IIIA, IIIB, IIIC, and IIID are 1:100,000, 1:200,000, 1:1,500,000, and 1:1,000,000, respectively [1,3,4]. Although formal diagnostic criteria for MPS III have not been established yet, the condition can be suspected based on clinical findings and supportive laboratory or imaging findings. Clinical findings may include symptoms such as coarse facies, thick hair and hirsutism, hepatosplenomegaly, joint stiffness, language and motor delays, behavioral problems including hyperactivity and aggressive or defiant behaviors, hearing loss, sleep disturbances, intellectual disability, seizure, and progressive developmental regression. Fig. 1 shows serial photographs, according to age, of my patient with MPS IIIB [5].
Fig. 1.
Serial photographs of a patient with MPS IIIB. (A) Mild facial coarseness was noted at the age of 4 years. Facial dysmorphism, including coarse facial features, a depressed nasal bridge, prominent eyebrows, and malocclusion, was apparent at the ages of (B) 13 and (C) 18 years. (D) He was bedridden and malnourished at the age of 21 years.
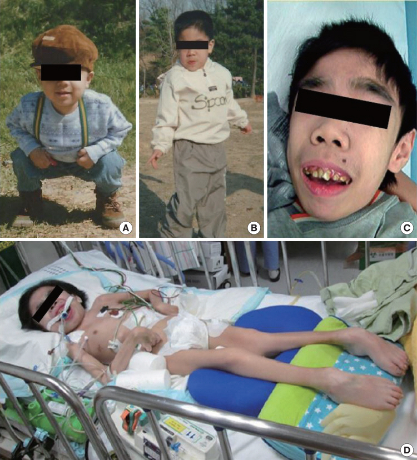
Supportive laboratory findings include an abnormality in either quantitative or qualitative GAG analysis, while imaging findings include a skeletal survey revealing mild signs of dysostosis multiplex such as thickened ‘oar-shaped’ ribs, scoliosis, kyphosis, misshaped or hypoplastic vertebral bodies, thickened diploic space, etc. The diagnosis of MPS III is established as either biallelic pathogenic variants in one of four genes (GNS, HGSNAT, NAGLU, and SGSH) or identification of deficiency of the respective lysosomal enzymes [1].
There is still no definitive treatment for the CNS symptoms of MPS III. The most difficult problem in treating neurological symptoms in MPS III patients is getting the drug into the brain, in other words, overcoming the blood brain barrier (BBB). Extensive research is being carried out on this problem.
This review describes possible strategies to overcome the BBB and recent therapies under investigation for MPS III.
The structure of BBB and transport through the BBB
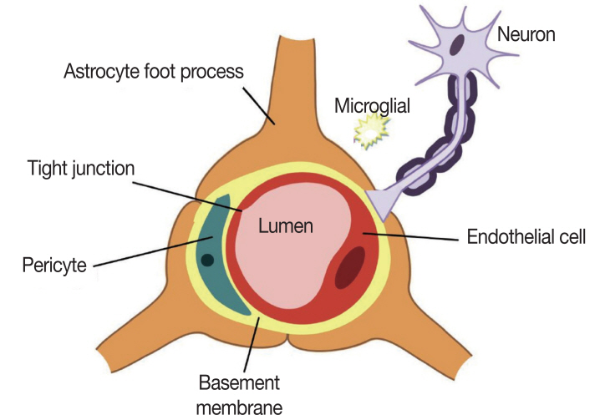
The BBB is a semi-permeable membranous barrier that is located at the interface between the blood and cerebral tissue. It consists of a complex system of endothelial cells, astrocyte foot processes, pericytes, microglia, and neurons (Fig. 2).
Fig. 2.
Angioarchitecture of the blood brain barrier (BBB). BBB consists of a complex system of endothelial cells, astrocyte foot processes, pericytes, microglia, and neurons.
In general, the BBB allows the passage of only lipophilic molecules with a low molecular weight (under 400-600 Da) and with a positive charge. Other molecules require certain cell endogenous transport systems, such as carrier-mediated, receptor-mediated or absorption-mediated transport. Commonly, there are five basic mechanisms by which solute molecules move across membranes: 1) simple or passive diffusion, e.g., blood gases, anesthetics, and heroin, 2) solute carriers, which constitute a superfamily of membrane transport proteins, e.g., glucose, amino acids, nucleosides, monocarboxylates, organic anions and cations, and L-3,4-dihydroxyphenylalanine 3) carrier-mediated efflux (efflux transporters), these ATP-binding cassette transporters use ATP hydrolysis to pump molecules across the membrane and therefore they can force the efflux of solutes against a concentration gradient. e.g., P-glycoprotein (Pgp:ABCB1) and breast cancer-related protein (BCRP:ABCG2) 4) receptor-mediated transcytosis, e.g., iron, insulin, and leptin, and 5) diapedesis of mononuclear leukocytes. Once the leukocytes enter the brain, they become microglia, which are cells with the brain's immune capabilities [1,6]. It has been estimated that more than 90% of all small-molecule drugs and nearly 100% of all larger therapeutics are not able to overcome the BBB [7]. Therefore, a lot of research effort is required to develop a new strategy that can effectively surpass the BBB and deliver therapeutic products into the CNS. The strategic treatment approaches to bypass the BBB can be categorized as invasive or non-invasive techniques (Table 2).
Table 2.
Techniques for brain drug delivery
| Invasive techniques |
|---|
| Blood–brain barrier transient disruption |
| Intracerebroventricular and intrathecal infusion |
| Non-invasive techniques |
| Modification of the drug to enhance its lipid solubility |
| Use of transport/carrier systems |
| Inhibition of efflux transporters that impede drug delivery |
| Trojan horse approach |
| Chimeric peptides |
| Monoclonal antibody fusion proteins |
| Pro-drug bioconversion strategies |
| Nanoparticle-based technologies |
| Gene therapy |
| Intracerebral gene therapy |
| Intranasal drug delivery |
| Substrate reduction therapy |
Techniques for brain drug delivery
Invasive techniques
- BBB transient disruption: This technique uses noxious agents, hyperosmotic solutions or ultrasound (mannitol, dimethyl sulphoxide, ethanol, metals, glycerol and polysorbate-80, X-irradiation, etc.) to shrink the brain’s endothelial cells by breaking down tight junctions, thus allowing various molecules to enter the cerebral tissue.
- Intracerebroventricular and intrathecal infusion: This technique uses injection or intraventricular infusion of therapeutic proteins directly into the cerebrospinal fluid (CSF). Animal studies on MPS IIIA show that continual infusion of replacement enzyme partially ameliorates clinical, histological, and biochemical aspects in MPS IIIA mice, when treatment begins at an early symptomatic stage [8]. Clinical trials with the same technique have been initiated in humans. Recombinant human heparan-N-sulfatase (rhHNS) administration via implanted intrathecal drug delivery device in twelve patients with MPS IIIA was found to be generally safe and well tolerated. The treatment resulted in a consistent decline of HS in the CSF, suggesting in vivo activity in the relevant anatomical compartment [9].
Non-invasive techniques
- Modification of the drug to enhance its lipid solubility: This technique involves chemical transformation of water-soluble molecules into lipid-soluble molecules that are capable of crossing the BBB [10].
- Use of transport/carrier systems: This technique involves chemical modification of small-molecule therapeutic drug to enable the use of endogenous transport/carrier systems that mimic the structure of related specific endogenous molecules. Glucose transporter type 1, large neutral aminoacid transporter type 1, cationic amino-acid transporter type 1, monocarboxylic acid transporter type 1, and equilibrative nucleoside transporter 1 are some of the most common endogenous carrier-mediated BBB transporters used as carrier systems for drug delivery [11].
- Inhibition of efflux transporters that impede drug delivery: This technique involves the pharmacological inhibition of select efflux transporters that prevent blood-to-brain drug uptake, examples of which include P-glycoprotein, breast cancer resistance protein (BCRP in humans and Bcrp in rodents), and multidrug resistance proteins (MRPs in humans and Mrps in rodents), etc. Among substrates transported by BCRP/Bcrp are chemotherapeutic agents (i.e., mitoxantrone), anthracyclines (i.e., etoposide and teniposide), and campothecin derivatives (i.e., topotecan and irinotecan). Transport of etoposide, cisplatin, and doxorubicin has been demonstrated though MRP6/Mrp6 [2,6].
- Trojan horse approach: This technique uses molecules such as endogenous ligands or monoclonal antibodies that, while acting as molecular ‘trojan horses’, bind exofacial epitopes on BBB receptor-mediated transport systems, thus triggering internalization of the receptor and the attached drug. This technique is being used to ferry drugs, proteins, and non-viral gene medicines across the BBB [2, 6].
- Chimeric peptides: This technique is being used for the transport of therapeutic compounds that can only be transported at very low rates through the BBB. The technique enables brain penetration by redesigning biological drugs into monoclonal antibody fusion proteins (IgG fusion proteins). Research to evaluate the efficacy of this technique is being conducted in mouse models of neurological disorders, including Parkinson's disease, stroke, Alzheimer's disease, and lysosome storage disorders [7].
- Pro-drug bioconversion strategies: This technique consists of developing pro-drug compounds (also called proagents), which are therapeutically inactive agents that can cross the BBB and enter into the brain parenchyma. Upon reaching the target site, these compounds undergo enzymatic and/or chemical transformations and modify their structure, resulting in a biologically active form that is capable of exerting the desired pharmacological effect [12].
- Nanoparticle-based technologies: This technology is primarily based on the use of nanoscale technology for drug release in the brain; the delivery system uses a variety of nanoscale drug delivery platforms, including primarily lipid and polymer-based nanoparticles (NPs) [13]. Studies have shown the potential of NPs as effective drug carriers. SFor example, a study tested the effectiveness of laronidase surface-functionalized lipid-core nanocapsules for the treatment of MPS I; another study loaded arylsulfatase B onto poly(butyl cyanoacrylate) NPs to affect neurological manifestations such as spinal cord compression in MPS VI by delivery of the therapeutic enzyme across the BBB; a third study in two murine models of MPS I and MPS II aimed at assessing the g7-NP brain delivery capacity using a model drug (Albumin-fluorescein isothiocyanate conjugate) [14-16].
- Gene therapy: This technique consists of transferring recombinant DNA with therapeutic function directly into the cells of specific organs [17]. It is a promising solution for neurodegenerative conditions where the neuropathology has spread throughout the entire brain, thus requiring a global CNS gene delivery for effective treatment. Two types of applications of gene therapy are possible: ‘ex vivo’, which involves the collection of target cells, treatment with genetic engineering techniques, and reinjection into the patient; and ‘in vivo’, which is the delivery of a gene directly into the body through a suitable vector such as a plasmid or a non-pathogenic viral vector [retrovirus, adenovirus, adeno-associated virus (AAV)]. Extensive studies of intravenous vector administration performed in newborn mice models of MPS I and MPS VII have shown encouraging results [18]. In another study, the use of systemic delivery AAV vectors in murine models of MPS I, IIIA, IIIB, and VI has demonstrated a reduction of the corresponding substrate in the CNS [19].
- Intracerebral gene therapy: This technique involves direct viral gene delivery into the brain parenchyma or ventricular system. A variety of non-neuronal viral vectors have been studied for CNS gene transfer in vivo in the brain of MPS I, MPS IIIA, MPS IIIB, and MPS VII mouse models via AAV, adenovirus, and lentivirus. The results of phase I/II clinical trials carried out to evaluate the feasibility and safety of brain injection of AAV vectors for MPS IIIA and IIIB suggest that intraparenchymal delivery may be a realistic option for neuropathic MPS [20].
- Intranasal drug delivery: This technique allows rapid delivery of therapeutic molecules directly to the CNS within minutes by bypassing the BBB via a neural pathway connecting the nasal mucosa and brain [21]. Lipid-based NPs have been studied for intranasal drug delivery and this non-invasive strategic approach has been used to evaluate the therapeutic efficacy of neurological symptoms in MPS I disease. In laboratory experiments in mice, intranasal administration of an α-L-iduronidase-encoding AAV serotype 9 (AAV9) vector resulted in the diffusion of the enzyme to deeper areas of the brain and related reduction of tissue GAG storage materials in the brain [22].
Substrate reduction therapy: genistein (4',5,7-trihydroxyisoflavone)
Genistein is a natural isoflavone that occurs in many plants and is known to have a variety of biological activities, from serving as a plant estrogen to displaying antioxidant activity [23]. Genistein is an inhibitor of tyrosine-specific protein kinases. Genistein-mediated reduction in the synthesis of HS is mediated through the inhibition of epidermal growth factor receptors and other growth factor receptors that regulate the transcription of genes involved in HS synthesis. In addition, in vitro studies have also revealed anti-inflammatory effects of genistein on nerve cells [24]. There are several studies that have identified genistein as an effective treatment for MPS III. Genistein has been shown to effectively inhibit HS synthesis in cultured fibroblasts of patients [9]. In another report, continuous administration of high-dose genistein to MPS IIIB mice for 9 months caused a significant reduction in lysosomal storage, HS substrate, and neuroinflammation in the cerebral cortex and hippocampus, resulting in correction of the behavioral defects observed [25]. Piotrowska et al. [26] reported that in eight pediatric patients with Sanfilippo disease, treatment with genistein-rich soy isoflavone extract showed possible positive effects on inhibition or slowing down of behavioral and cognitive problems. de Ruijter et al. [27] reported that in thirty MPS III patients administered genistein-rich soy isoflavone extract, there was an effective reduction in urinary excretion of GAGs and plasma HS concentration.
Studies on MPS III are actively underway
In MPS IIIA patients, these include a phase IIb study involving intrathecal rhHNS [9], intravenous delivery of a chemically modified sulfamidase in MPS IIIA mice [28], a phase II/III study involving AAV10, and a phase I/II study involving AAV9. In MPS IIIB patients, a phase I/II study involving tralesinidase Alf, a phase II/III study involving anakinra (kineret), and a phase I/II study involving AAV9 and AAV5 are ongoing.
CONCLUSIONS
MPS III is a multisystem lysosomal storage disease that presents progressive CNS degeneration with no currently available treatment for the CNS symptoms. Recently, many studies have been conducted on the treatment of the CNS symptoms. Invasive and non-invasive technique strategies that allow drugs to pass through the BBB and reach the CNS are being tested and have proven effective. In addition, the application of genistein treatment as a substrate reduction therapy is currently in progress. Thus, a possible treatment for CNS symptoms of MPS III is expected in the future.
References
, (2019, updated on 2019 Sep 19) Mucopolysaccharidosis Type III. GeneReviews® Seattle: University of Washington https://www.ncbi.nlm.nih.gov/books/NBK546574/, Internet
- Submission Date
- 2020-10-03
- Revised Date
- 2020-10-13
- Accepted Date
- 2020-10-31
- Downloaded
- Viewed
- 0KCI Citations
- 0WOS Citations

